Ophthalmic pharmaceutical composition with improved preservative effectiveness or light stability
A technology of composition and medicine, which is applied in the field of enhancing the antiseptic effect of catiolol, can solve the problems of cost and stability of storage facilities unsuitable for long-term continuous application, and achieve the goal of enhancing antiseptic effect or antibacterial effect and improving photostability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
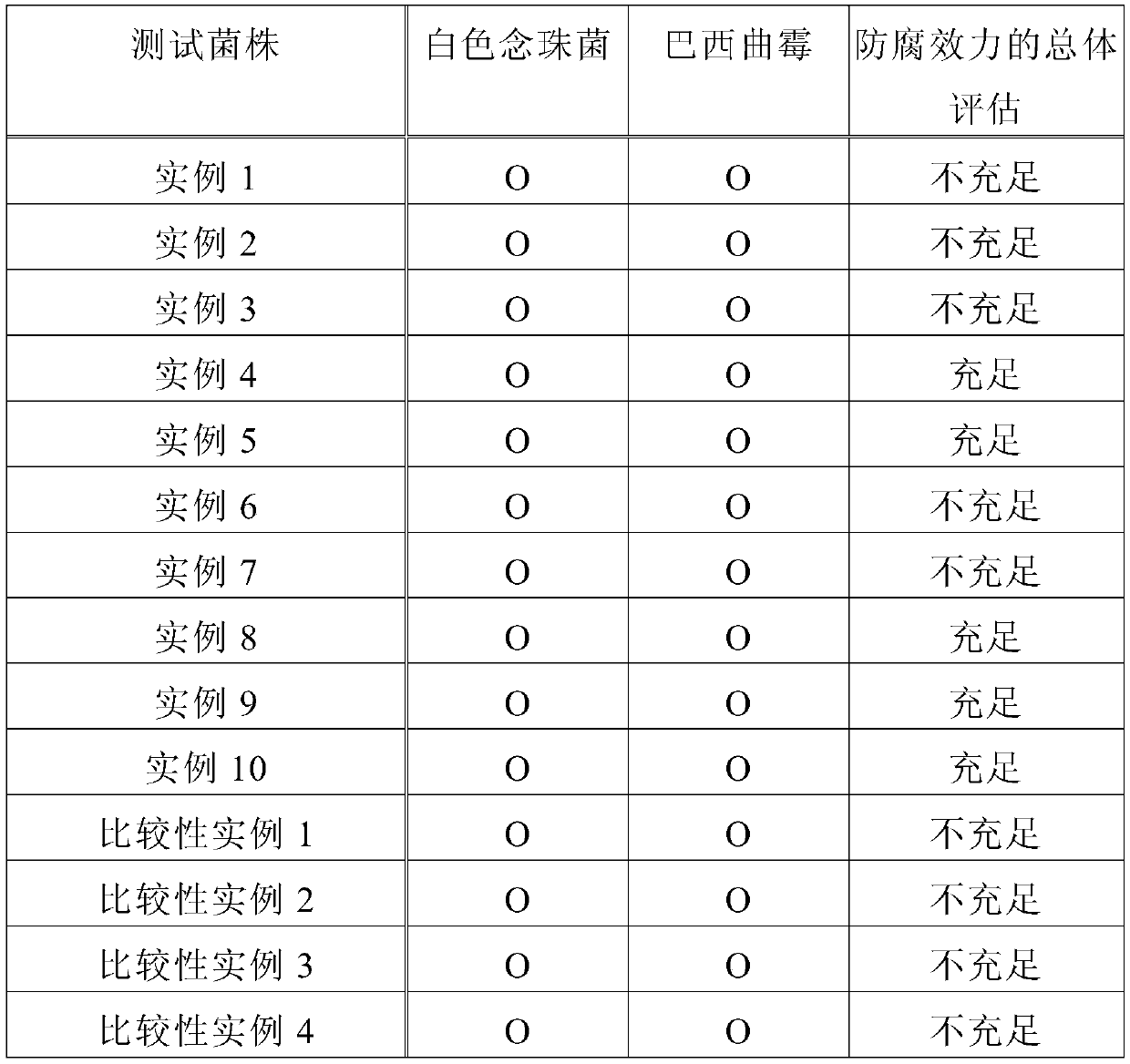
Examples
example 19 and 20
[0166]
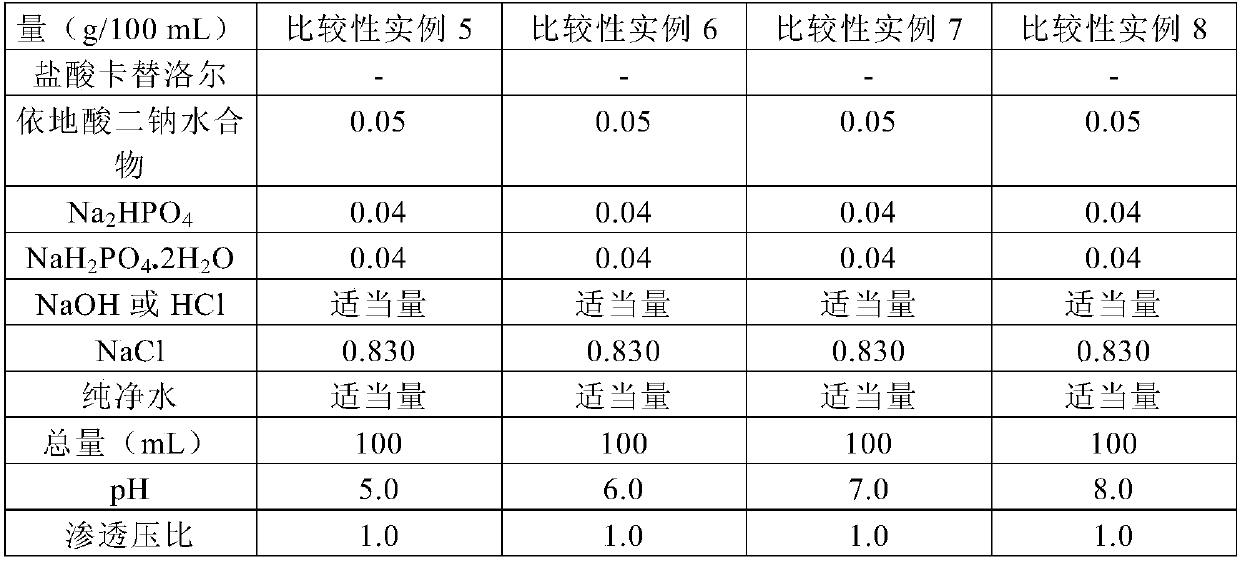
[0167] The components of the test solutions of Comparative Examples 19 and 20 are shown in Table 12. Carteolol hydrochloride, disodium hydrogen phosphate anhydrous and sodium dihydrogen phosphate dihydrate were mixed to prepare the compositions shown in Table 12. Add sterile purified water to the mixture to dissolve it. The pH of these solutions was adjusted to 6.7 by adding 5N sodium hydroxide, and sterile purified water was added to these solutions to obtain the prescribed volume. These solutions were filtered through a 0.22-μm membrane filter, and 5 mL of each solution was loaded into sterile glass containers to be used as test solutions.
[0168] [Table 11]
[0169]
[0170] [Table 12]
[0171] Quantity (g / 100mL)
Comparative Example 19
Comparative Example 20
Carteolol Hydrochloride
1.0
2.0
-
-
Na 2 HPO 4
0.04
0.04
NaH 2 PO 4· 2H 2 o
0.04
0.04
NaOH...
example 1
[0203] : Formulation example of a formulation comprising a prostaglandin F2α derivative
[0204] Examples 30 to 39 and Comparative Example 29 were prepared according to the following methods.
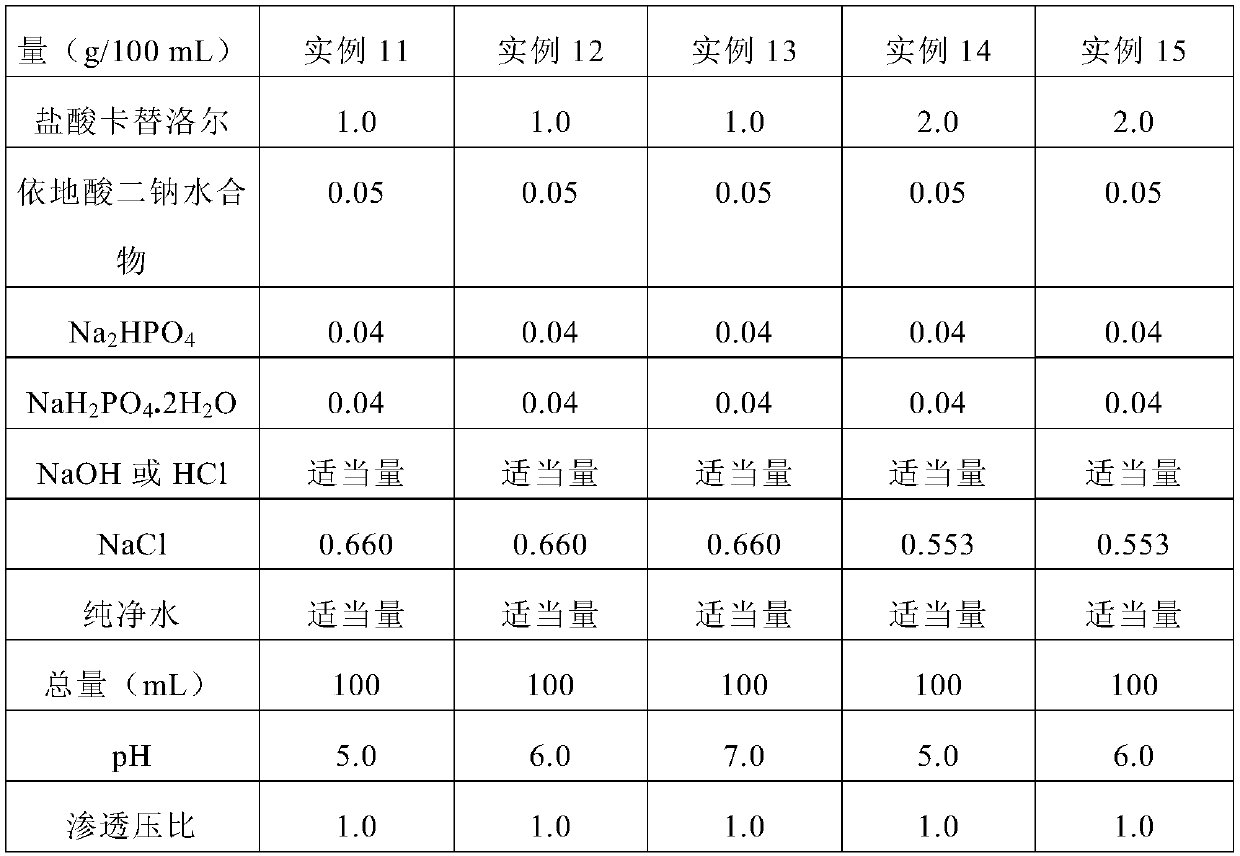
example 30
[0206] Latanoprost (0.005 g), polysorbate 80 (0.1 g) and purified water (80 g) were measured and mixed, and the mixture was warmed to 60° C. to dissolve it. Then, the mixture was cooled to room temperature. To this solution were added carteolol hydrochloride (2.0 g), alginic acid (1.0 g), boric acid (1.0 g) and edetate disodium hydrate (0.1 g). The mixture was dissolved by adding sodium hydroxide, and the pH of the mixture was adjusted to 6.5. Then, purified water was added to the mixture so that the total amount was 100 g. The solution was filtered through a membrane filter with a pore size of 0.2 μm to prepare Example 30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com