High-affinity monoclonal antibody against human copeptin, preparation method and application thereof
A monoclonal antibody and copeptin technology, applied in the field of biomedicine, can solve the problems of high operational difficulty, uncontrollable immunization dose, and high immunization cost, and achieve the effects of promoting effective maturation, shortening detection time, and increasing immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
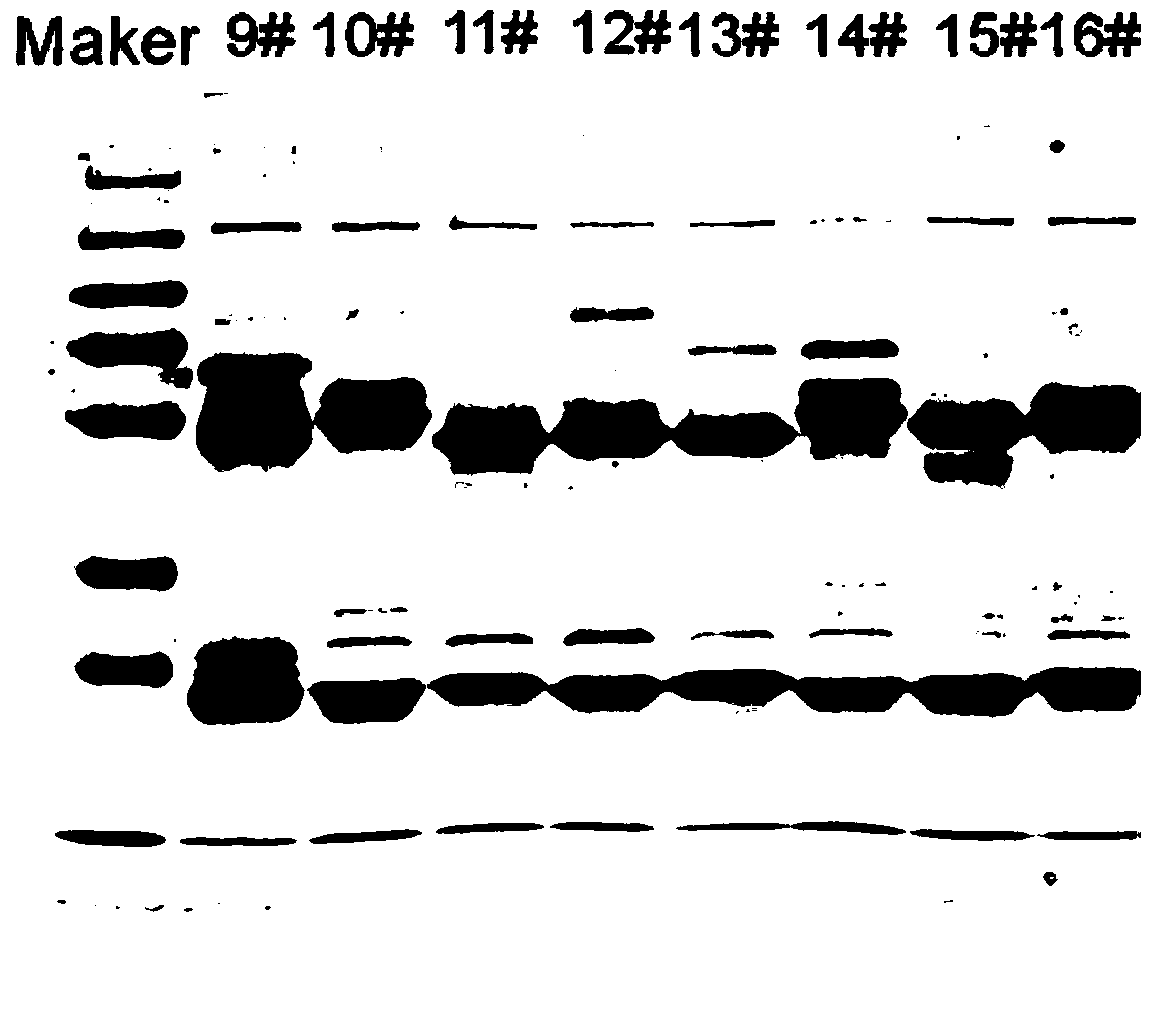
Examples
preparation example Construction
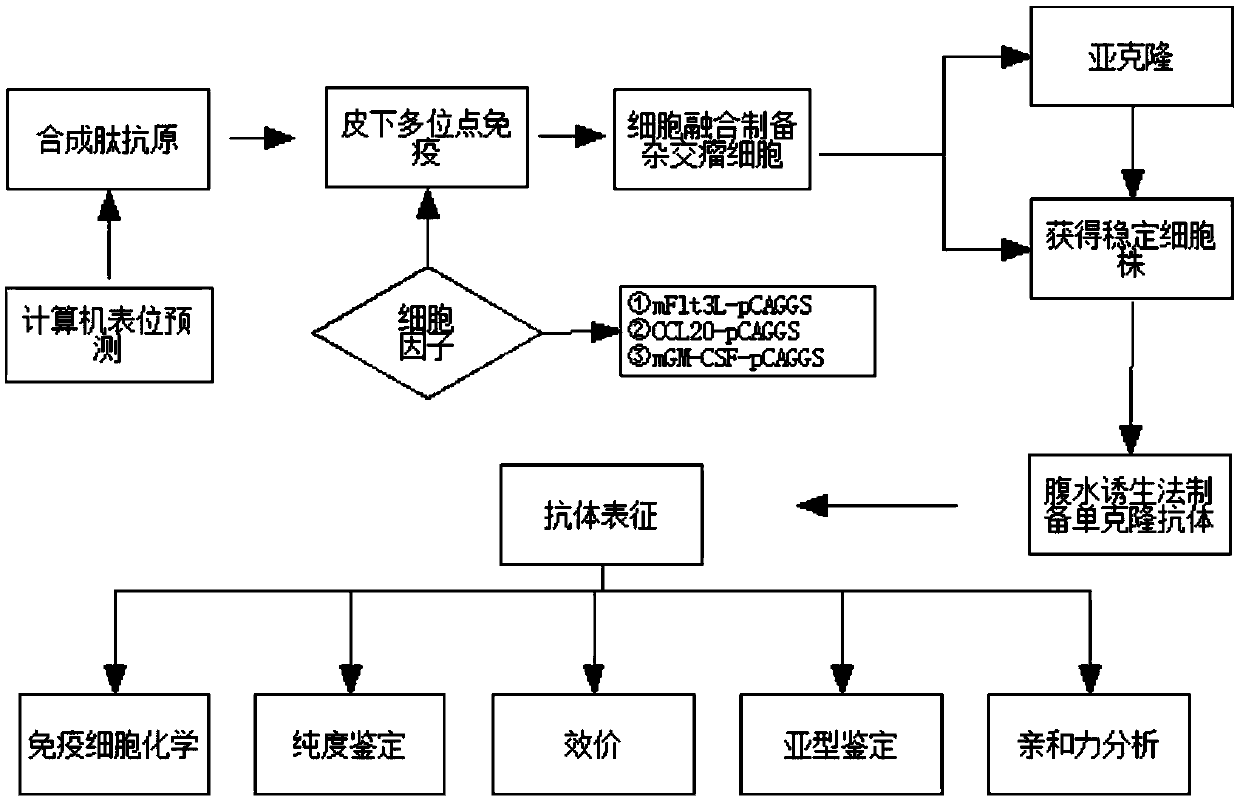
[0038] A preparation method of hybridoma cells secreting anti-human copeptin monoclonal antibody of the present invention comprises the following steps: immunizing mice with human copeptin antigen; delivering plasmids expressing cytokines to the immunized mice; collecting Splenocytes or lymph node cells of mice delivered by the plasmid, and the spleen cells or lymph node cells are fused with mouse tumor cells to obtain hybridoma cells.
[0039] Optionally, the copeptin antigen is a polypeptide comprising the amino acid sequence of SEQ ID NO:1, SEQ ID NO:2 or SEQ ID NO:3. Preferably, the copeptin antigen is a copeptin antigen coupled with a macromolecular protein, and the macromolecular protein is at least one of KLH, OVA, and BSA. More preferably, the copeptin antigen is a copeptin antigen with KLH coupled to the C-terminus.
[0040] In some of these embodiments, the cytokines include mFlt31, mGM-CSF and mCCL20. Preferably, the cytokine-expressing plasmid is pCAGGS.
[0041...
Embodiment 1
[0046] Example 1 Preparation method of high-affinity anti-human copeptin monoclonal antibody
[0047] 1.1 Polypeptide synthesis
[0048] Copeptin antigen polypeptides (cppF, N-cpp, C-cpp) were synthesized and coupled with KLH to prepare complete antigens to obtain KLH-cppF, KLH-N-cpp, KLH-C-cpp. The amino acid sequence is:
[0049] cppF: ATQLDGPAGALLLLRLVQLAGAPEPFEPAQPDAY (SEQ ID NO: 1),
[0050] N-cpp: CATQLDGPAGALLLLRLV (SEQ ID NO: 2),
[0051] C-cpp: CLAGAPEPFEPAQPDAY (SEQ ID NO: 3),
[0052] That is, the complete antigen obtained by coupling KLH is:
[0053] KLH-cppF(KLH-C-ATQLDGPAGALLLLRLVQLAGAPEPFEPAQPDAY),
[0054] KLH-N-cpp (KLH-C-ATQLDGPAGALLLLV),
[0055] KLH-C-cpp (KLH-C-LAGAPEPFEPAQPDAY);
[0056] Peptide synthesis and coupling adopt conventional methods, entrusted to Jill Biochemical Shanghai Co., Ltd. to complete. The purity was determined by high performance liquid chromatography (HPLC), all >95%.
[0057] 1.2 Plasmid construction
[0058] The pCAGGS v...
Embodiment 2
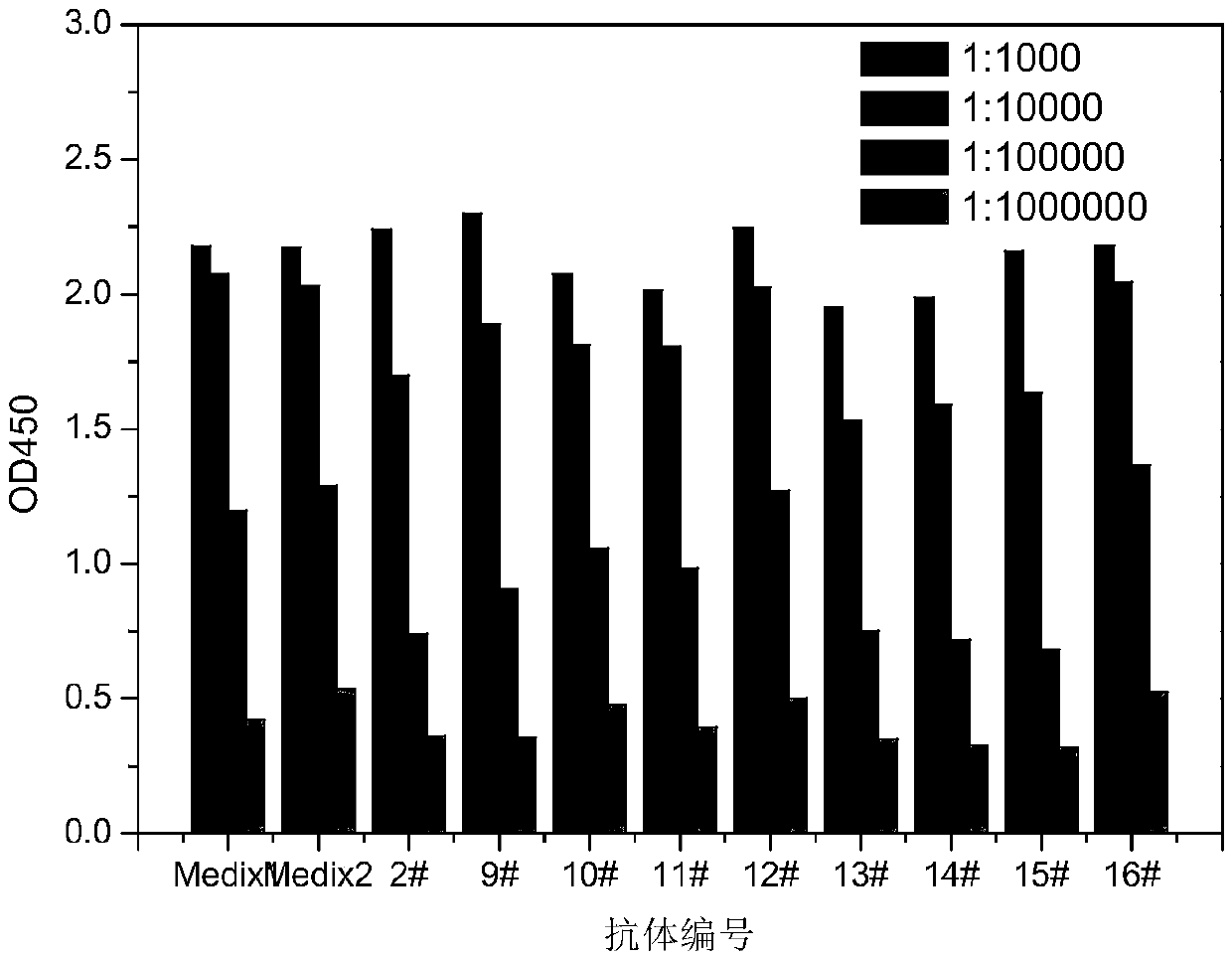
[0073] Example 2 Immune Effect and Antibody Titer Measurement
[0074] Cytokine group: the immunization strategy described in Example 1: the tail vein injection of cytokines was performed one week after each subcutaneous immunization.
[0075] Control group 1: Compared with Example 1, cytokine tail vein injection was not performed after each immunization, and the remaining steps and methods were the same as Example 1.
[0076] Control group 2: Compared with Example 1, after the initial immunization of subcutaneous site injection, the plasmids expressing cytokines: pCAGGS-mFlt3L and pCAGGS-mGM-CSF were injected through the tail vein one week later, that is, compared with the experimental group, pCAGGS-mCCL20 was not injected.
[0077] The comparison results of the immune effects of the above two groups are shown in Table 1.
[0078] Table 1 Comparison of immune effects
[0079]
[0080]
[0081] It can be seen from Table 1 that the immune effect of the cytokine group, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com