Patents
Literature
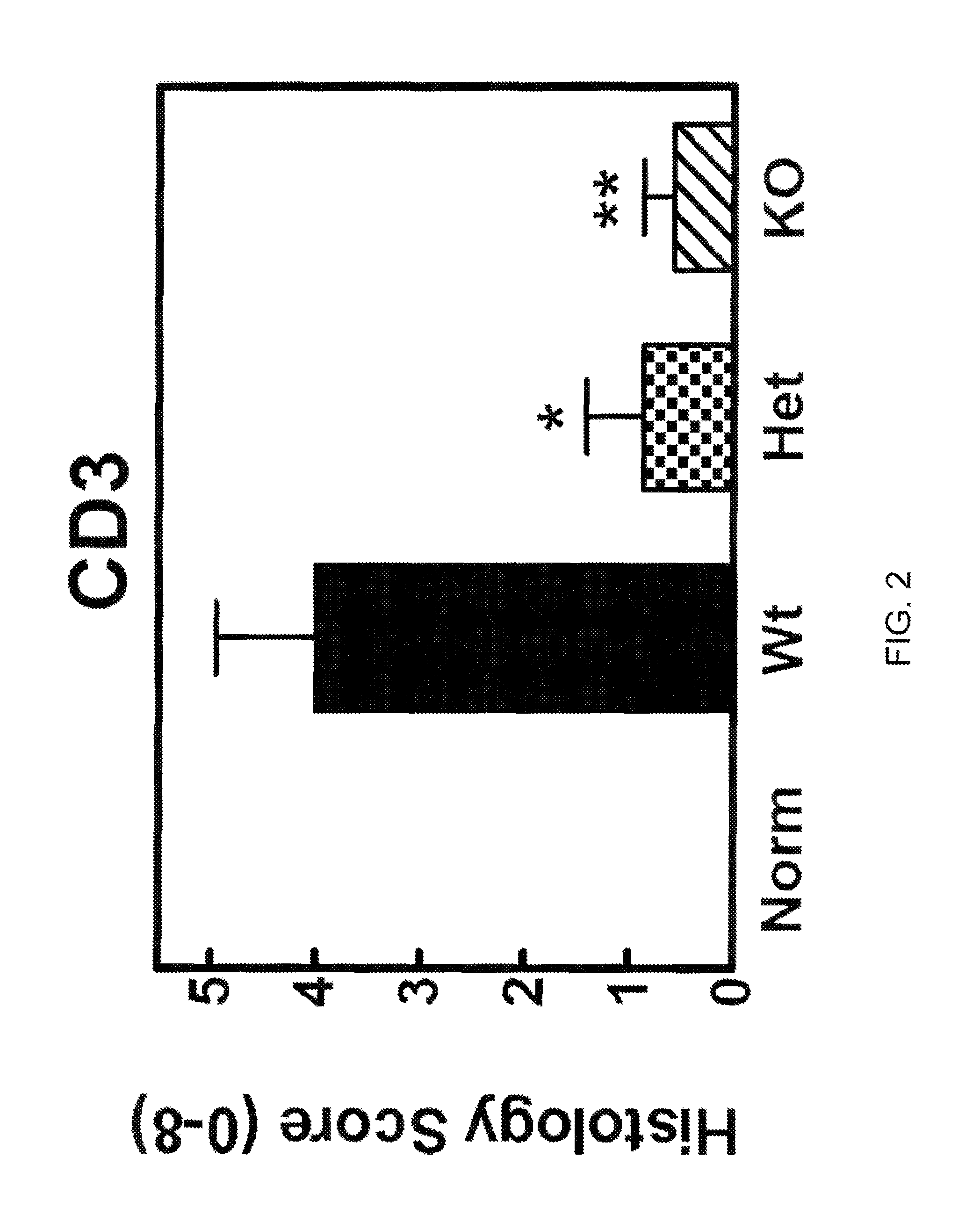
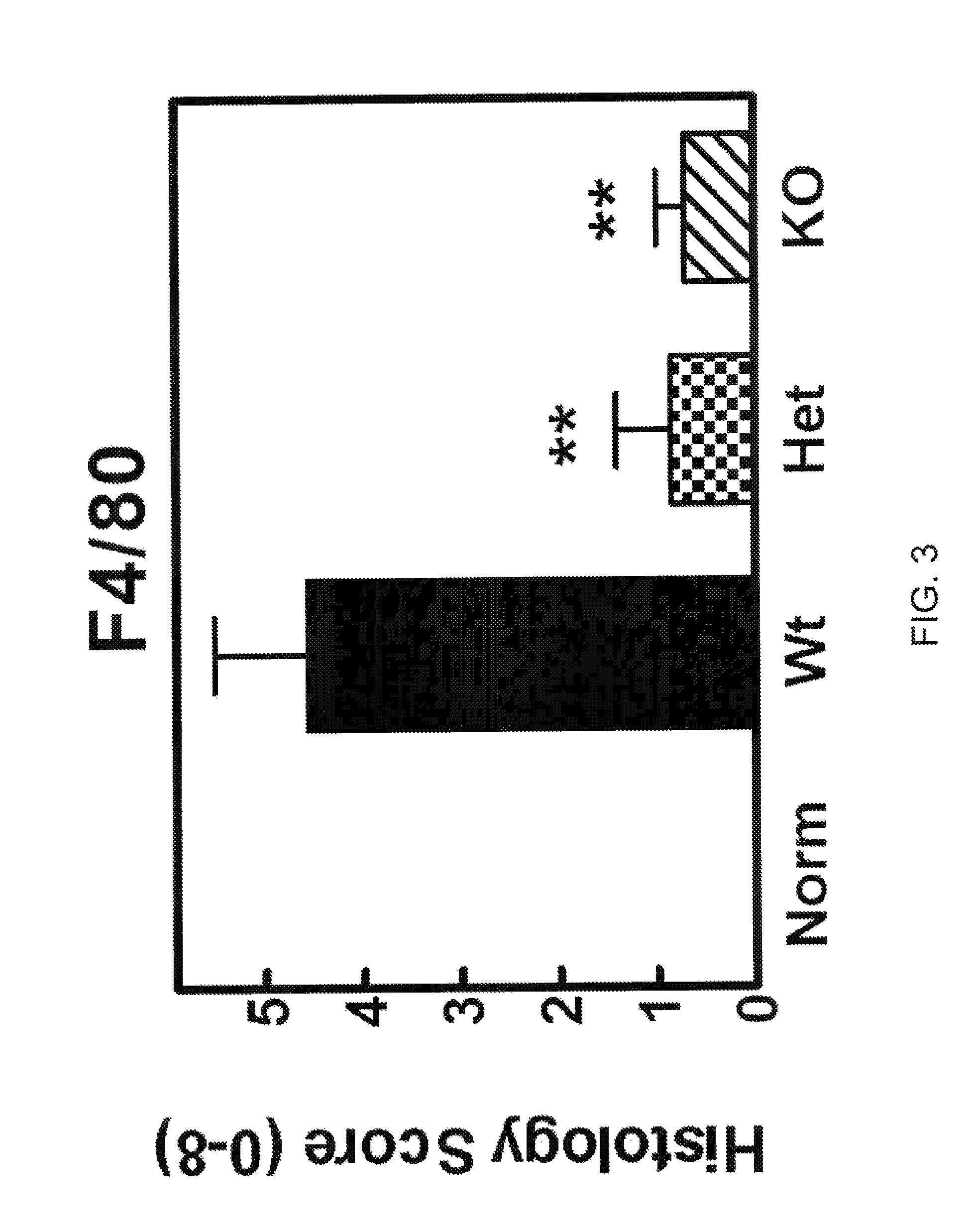
38 results about "CCL20" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chemokine (C-C motif) ligand 20 (CCL20) or liver activation regulated chemokine (LARC) or Macrophage Inflammatory Protein-3 (MIP3A) is a small cytokine belonging to the CC chemokine family. It is strongly chemotactic for lymphocytes and weakly attracts neutrophils. CCL20 is implicated in the formation and function of mucosal lymphoid tissues via chemoattraction of lymphocytes and dendritic cells towards the epithelial cells surrounding these tissues. CCL20 elicits its effects on its target cells by binding and activating the chemokine receptor CCR6.
Novel biomarker for liver cancer and applications for same
InactiveUS20110306513A1Improve accuracyImprove reliabilityPeptide librariesSugar derivativesLiver tissueOncology
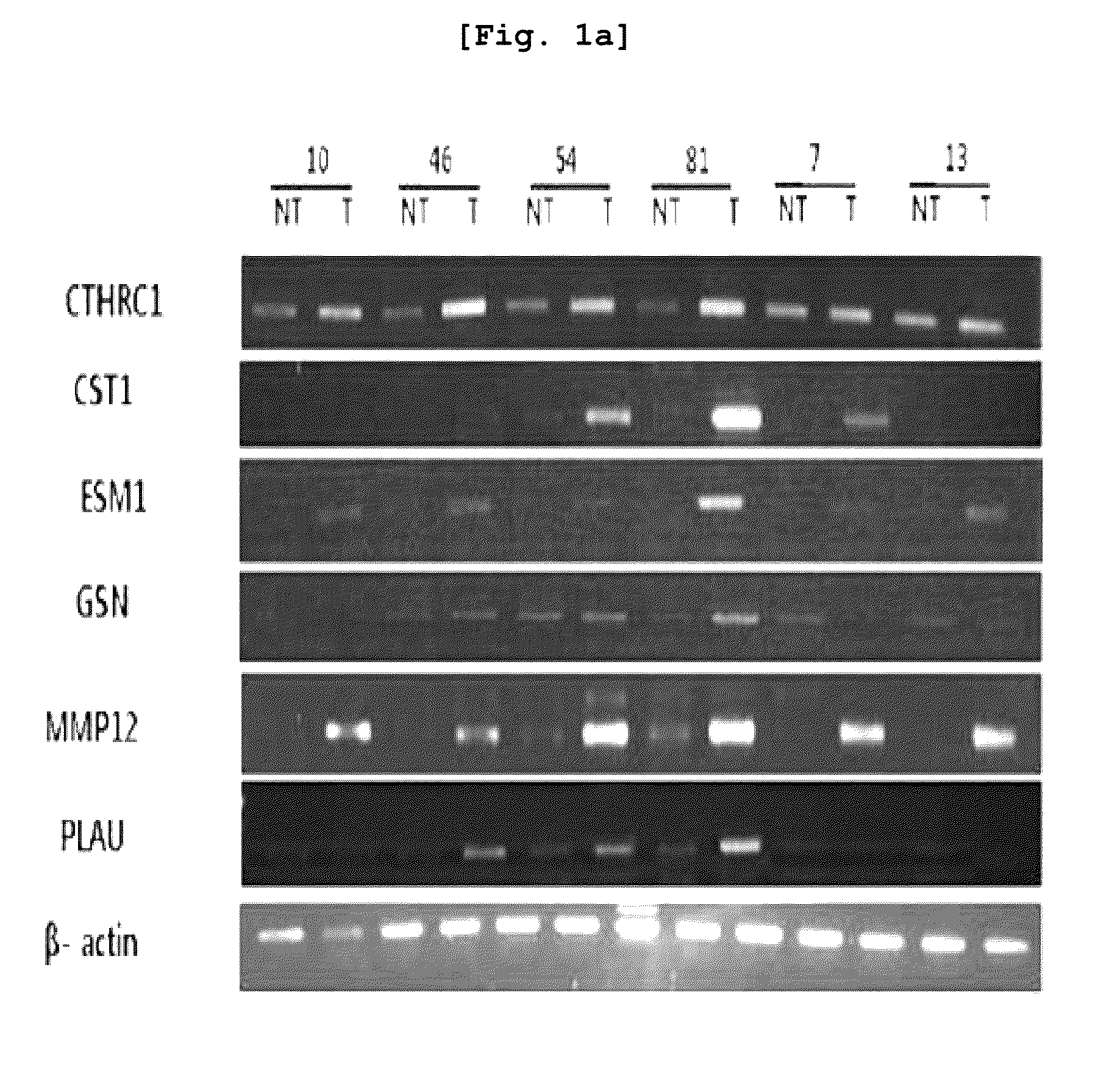
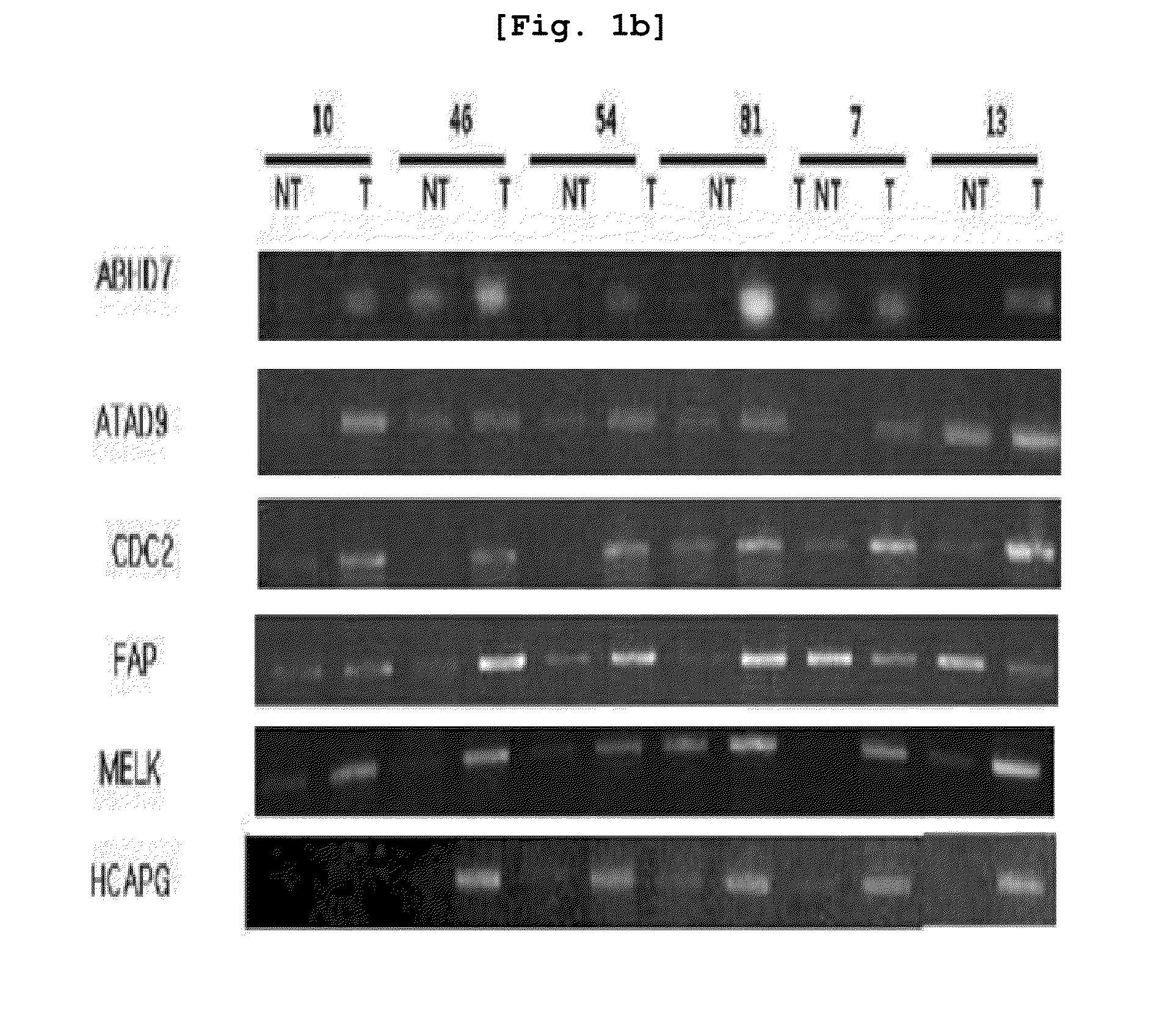
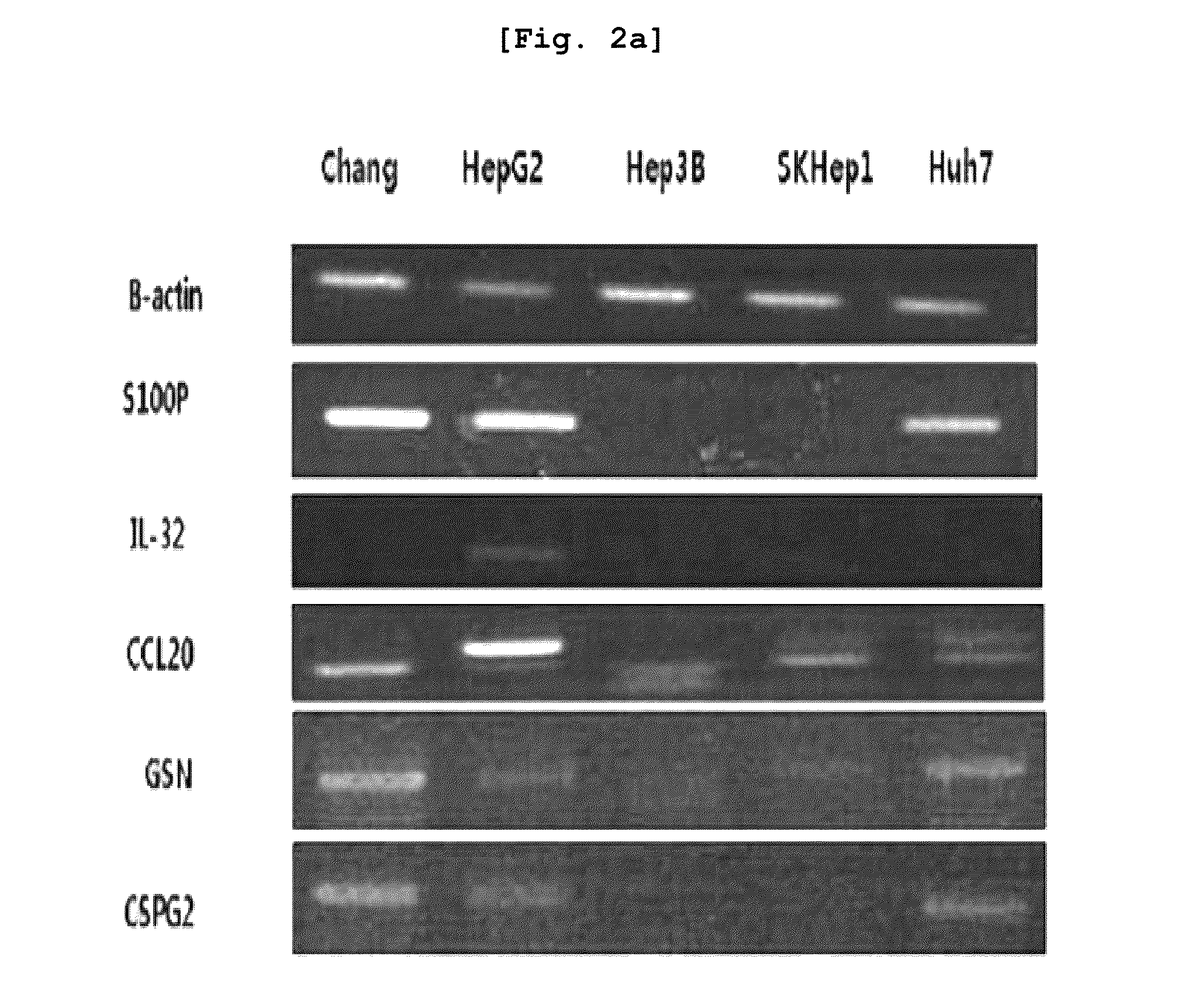
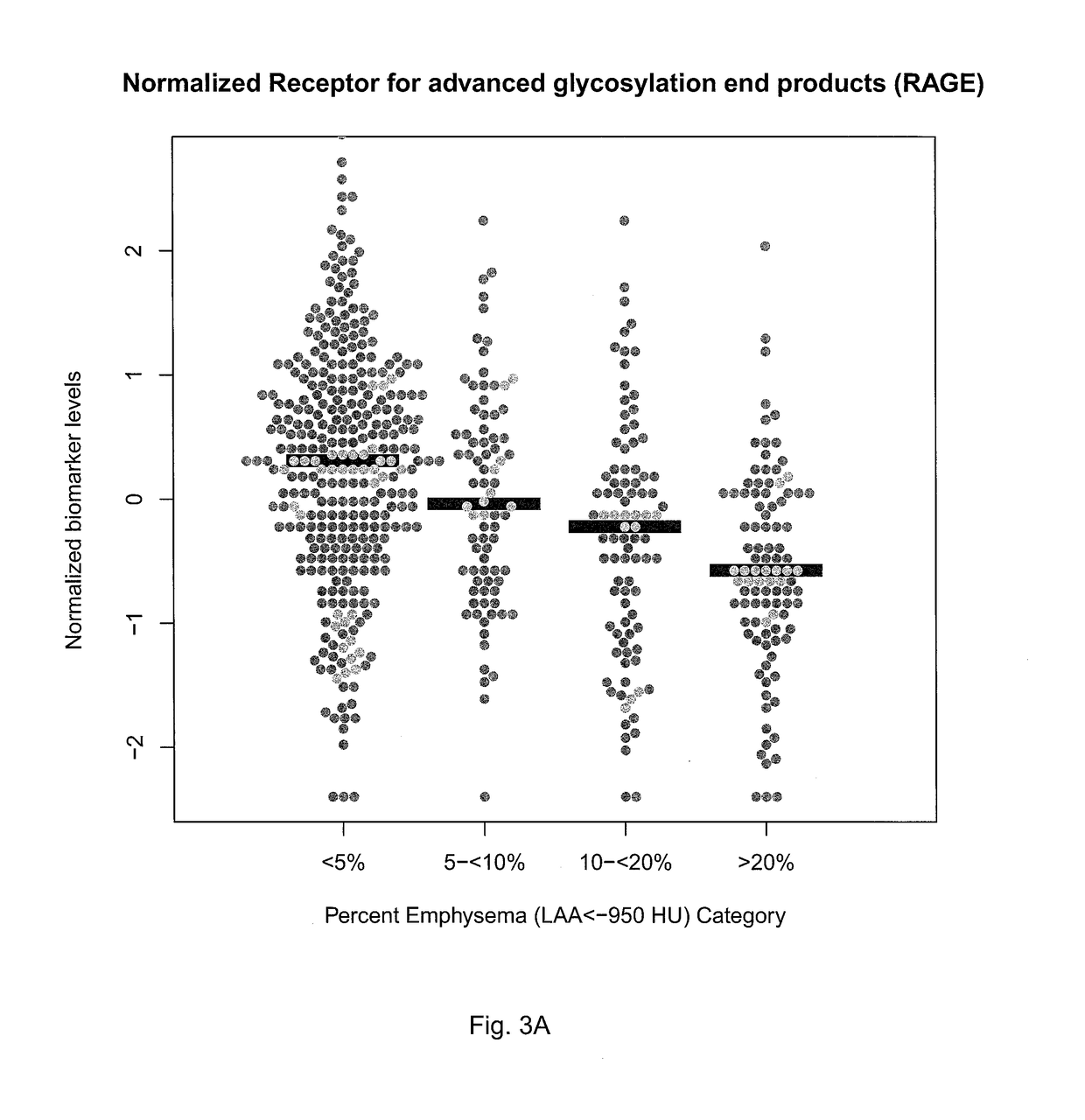
The present invention relates to the elucidation of a gene that can act as a novel marker for liver cancer diagnosis and to diagnostic and prognostic measurements of liver cancer using the same. More specifically, it relates to a diagnosis kit that enables diagnostic and prognostic measurement of a liver cancer using a preparation that measures expression levels of at least one gene selected from a group of liver cancer diagnosis markers consisting of S100P, NK4, CCL20, CSPG2, PLAU, MMP12, ESM-1, ABHD7, HCAPG, CXCL-3, Col5A2, MAGEA, GSN, CDC2, CST1, MELK, ATAD2, FAP and MSN and / or a method for diagnostic and prognostic measurement of liver cancer using the same. These have been discovered using normal liver tissues and liver cancer tissues collected from the same liver cancer patient of the present invention and represent the markers whose accuracy and reliability have been greatly improved as markers of liver cancer. The markers of the present invention can be used for the accurate diagnosis and prognosis of liver cancer.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Method to diagnose or screen for inflammatory diseases
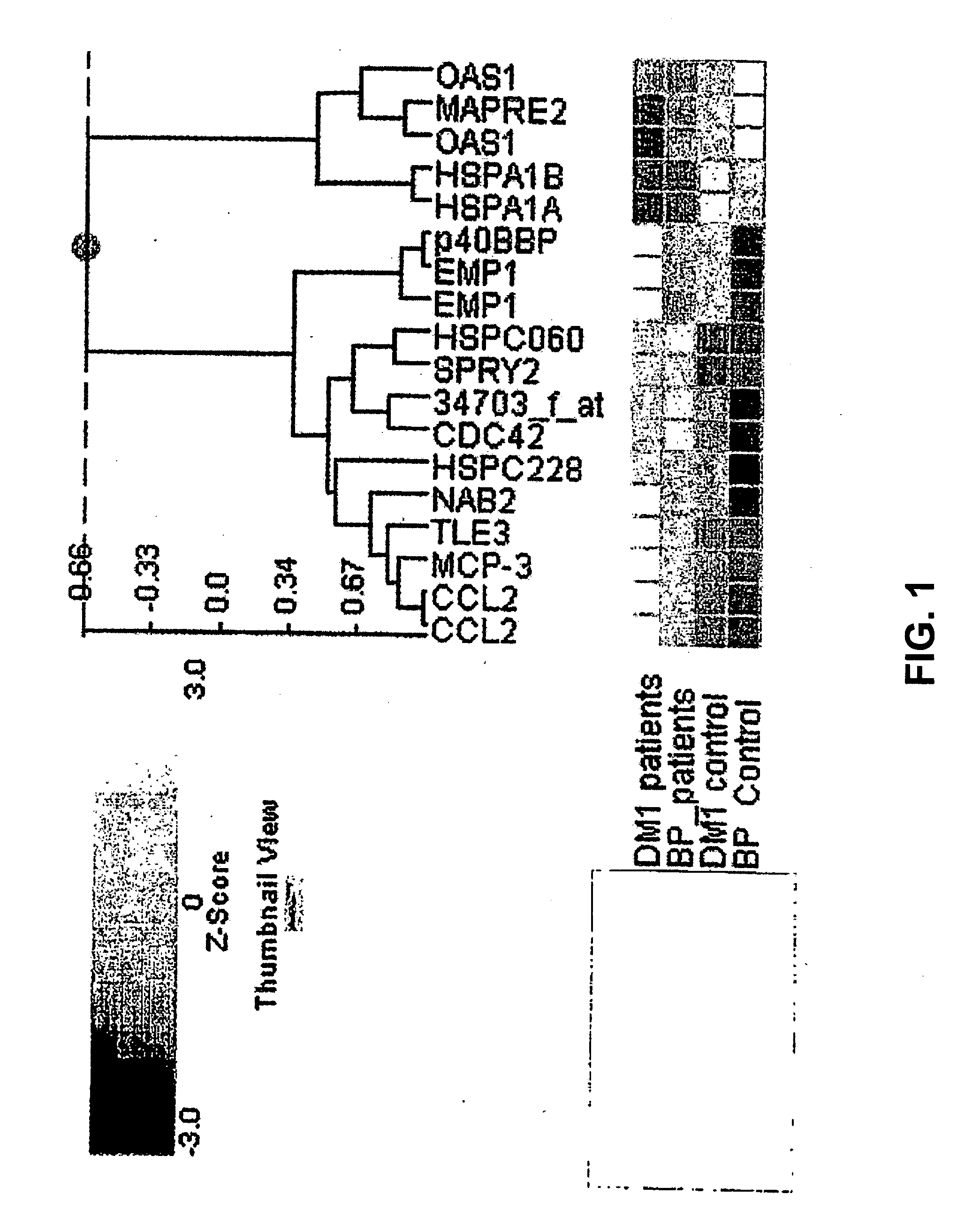
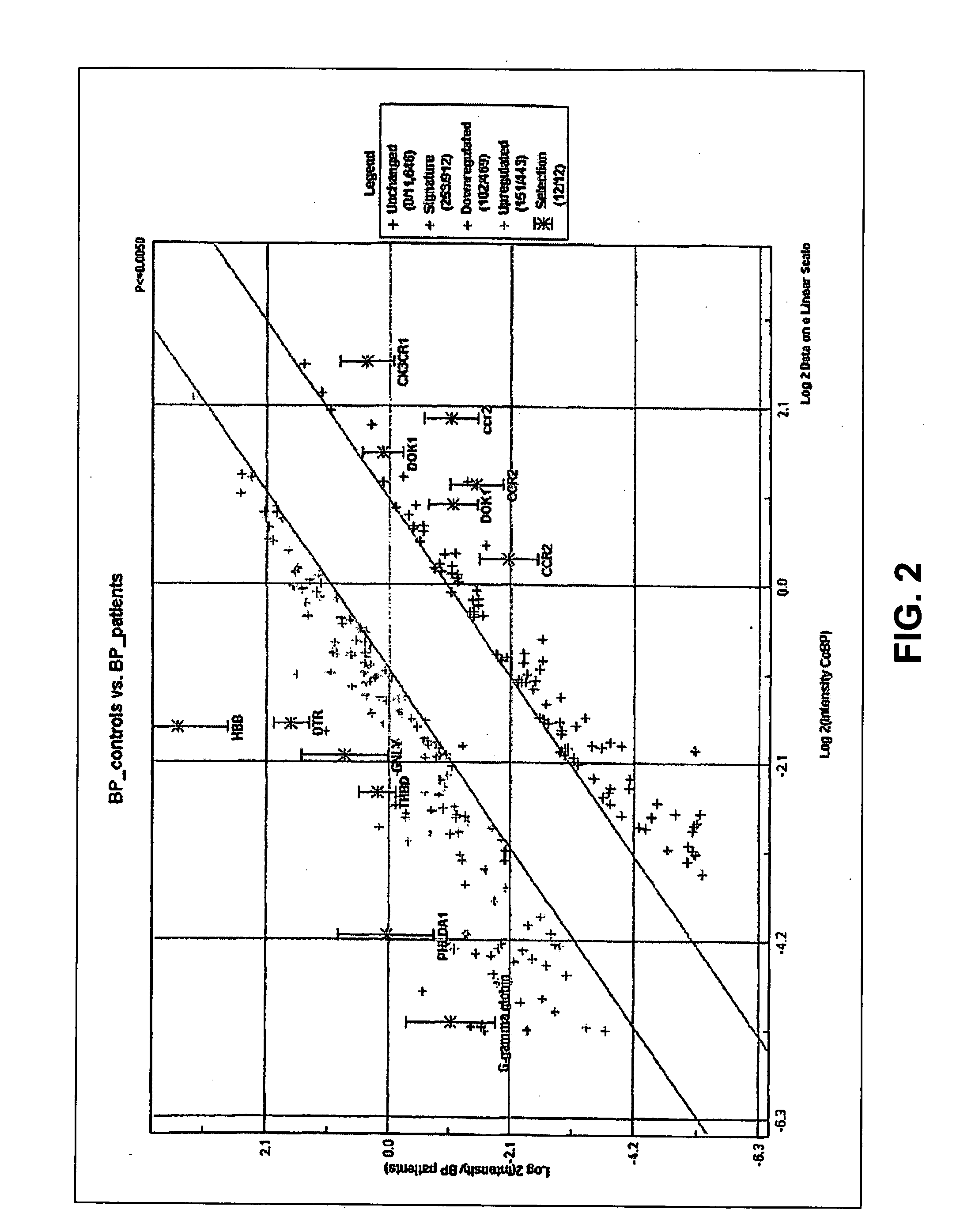
The invention relates to the field of medical diagnostics. More specifically, the invention relates to methods to diagnose or screen for inflammatory conditions or disease, including auto-inflammatory disease and affective disorder, in a subject, preferably a human subject, by assaying for a marker for an inflammatory disease. Provided is a method to diagnose, screen for or predict the development of an affective disorder (AD), preferably bipolar disorder (BP), in a subject, the method comprising determining the level of at least one, preferably at least two, more preferably at least three, most preferred at least four, AD-specific gene product(s) in a biological sample isolated from the subject, preferably peripheral blood monocytes, wherein the gene is selected from the group comprising ATF3, phosphodiesterase 4 B, CXCL2, BCL2-related protein A2, Dual specificity phosphatase 2, TNFα-induced protein 3 / A20, BTEB1 CXCL3, Chemokine CCL-3 like, CCL-4, CCL20, CX2CR1, Amphiregulin, Thrombomodulin, Heparin-binding EGF-like growth factor, DNA-damaged inducible transcript, V28 chemokine-like receptor, TRAIL. MAPK6, B4BP4, PBEF1, Thrombospondin 1, MAFF, HSP70, CCL2, MCP-3, CCR2, CX3CR1, DOK1, HBB, G-gamma globin, THBD, PHLDA1, DTR and GNLY.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Neutralizing Anti-ccl20 antibodies
ActiveUS20120148592A1Reduces human CCL20-induced chemotaxisShorten the progressAntibacterial agentsFungiDiseaseHeavy chain
The present invention relates to novel humanized, chimeric and murine antibodies that have binding specificity for the human CC chemokine ligand 20 (CCL20). The present invention further relates to heavy chains and light chains of said antibodies. The invention also relates to isolated nucleic acids, recombinant vectors and host cells that comprise a sequence which encodes a heavy chain and / or a light chain of said antibodies, and to a method of preparing said antibodies. The anti-CCL20 antibodies of the invention can be used in therapeutic applications to treat, for example, inflammatory and autoimmune disorders and cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
Methods and compositions for the treatment and prevention of cancer
ActiveUS20090068234A1Organic active ingredientsTumor rejection antigen precursorsCancer preventionOncology
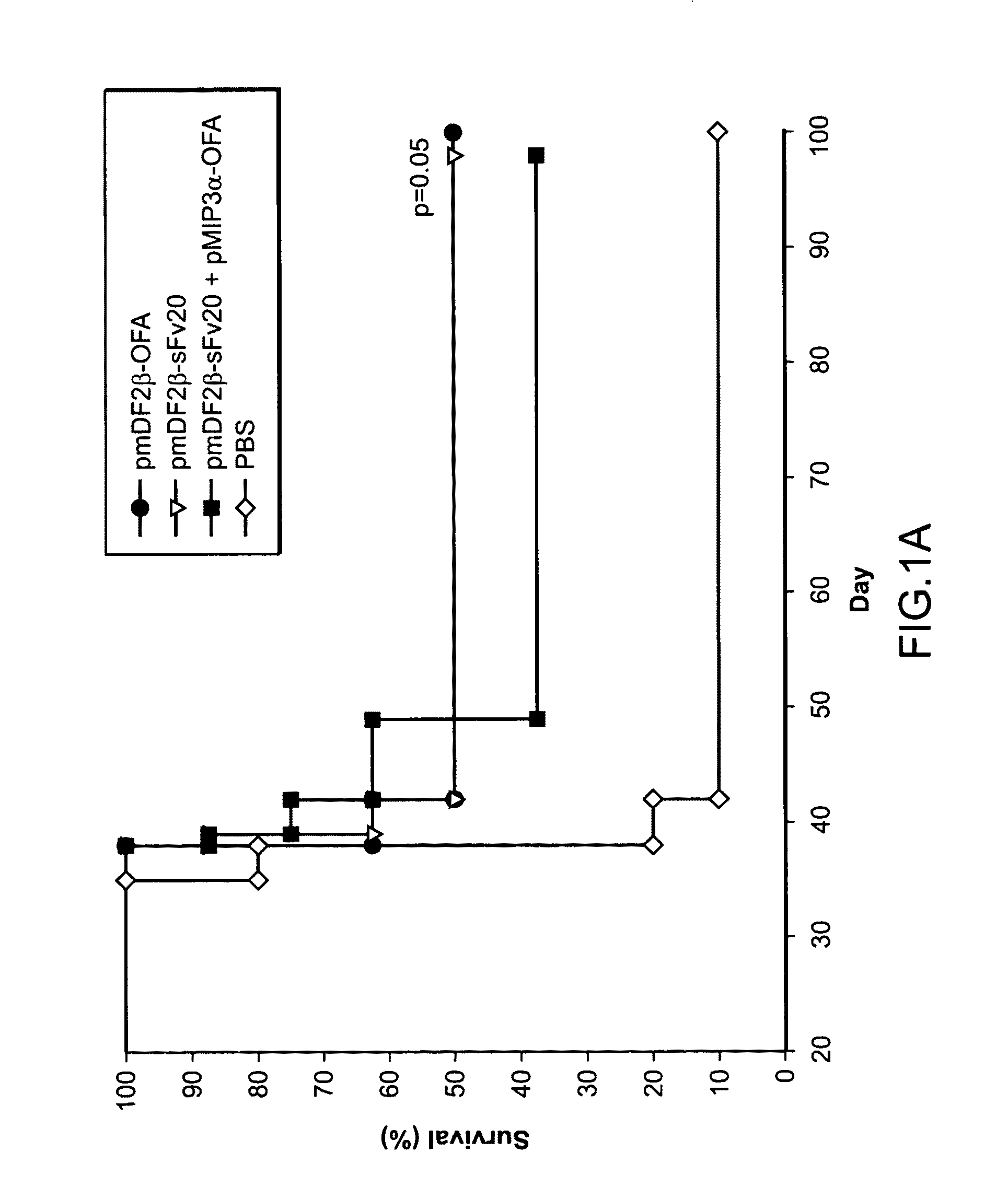
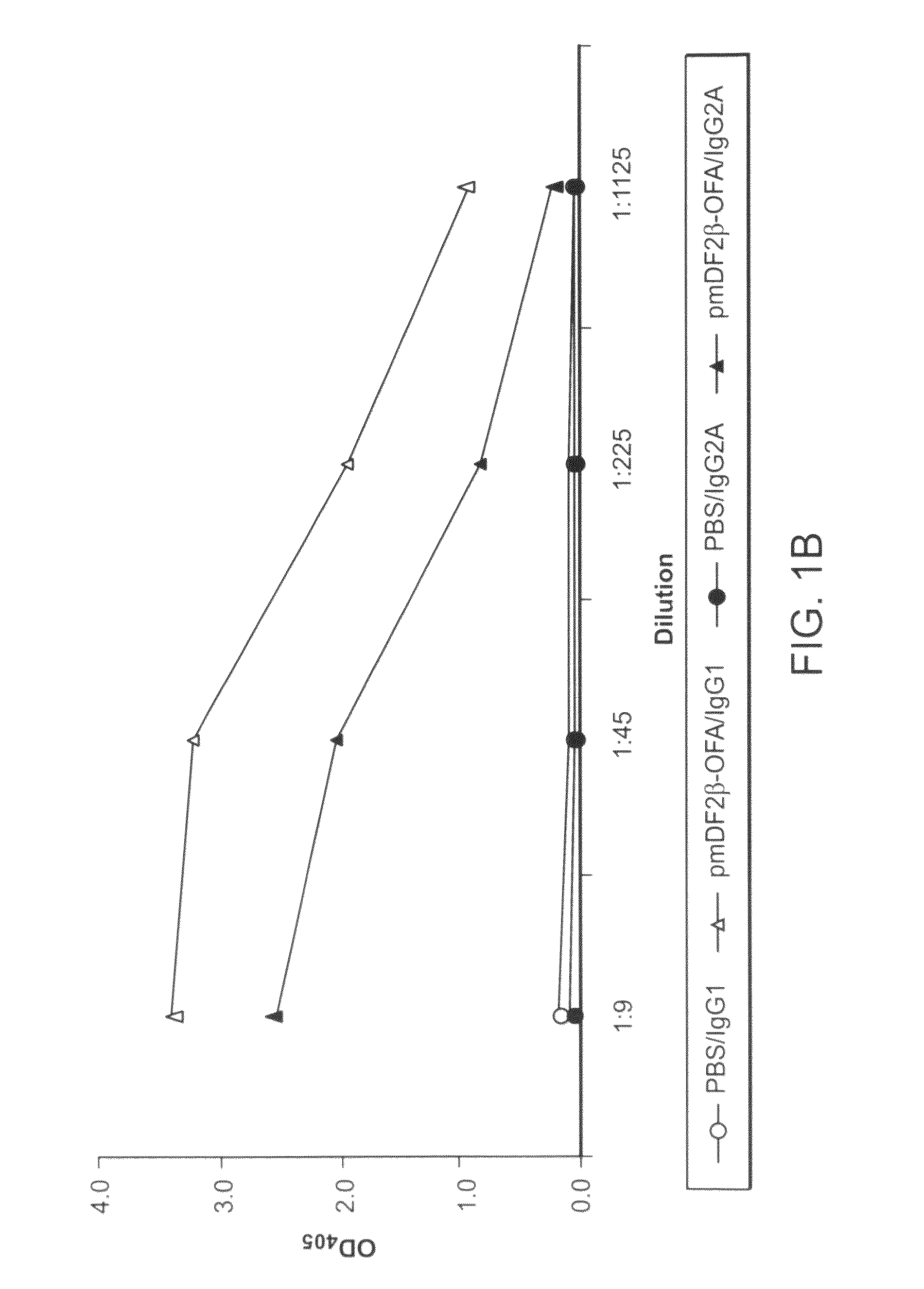
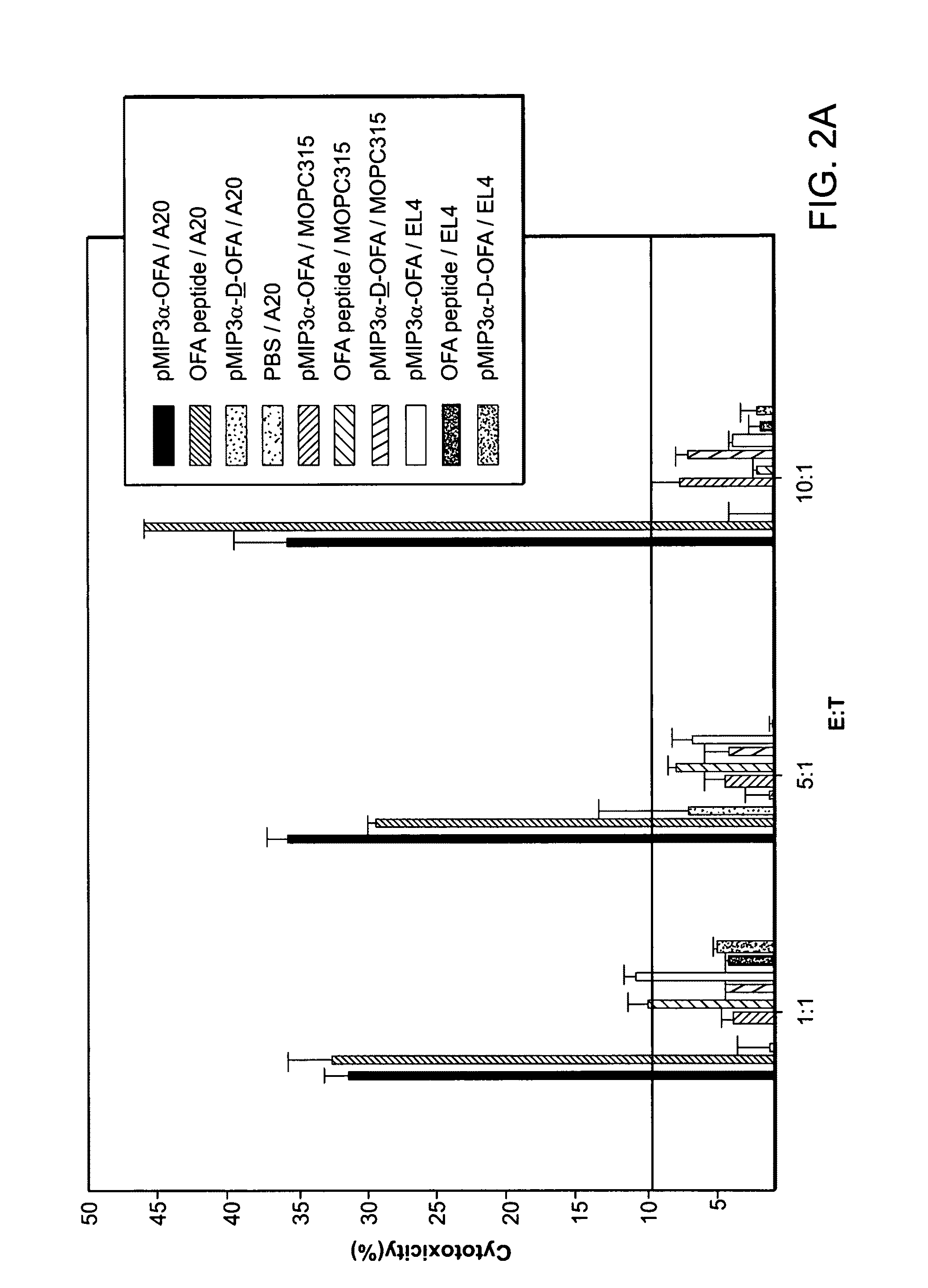
The instant invention provides compositions for the treatment of cancer. Specifically, the invention provides polypeptides and nucleic acid molecules comprising tumor-associated embryonic antigens, e.g., OFA-iLRP, and chemoattractant ligands, e.g., a proinflammatory chemokine such as MIP3α / CCL20 or β-defensin mDF2β. The invention further provides cancer vaccines and methods for treating subjects having, or at risk of developing, cancer.
Owner:UNITED STATES OF AMERICA
Neutralizing anti-CCL20 antibodies
ActiveUS8491901B2Reduces human CCL20-induced chemotaxisShorten the progressAntibacterial agentsBacteriaDiseaseHeavy chain
The present invention relates to novel humanized, chimeric and murine antibodies that have binding specificity for the human CC chemokine ligand 20 (CCL20). The present invention further relates to heavy chains and light chains of said antibodies. The invention also relates to isolated nucleic acids, recombinant vectors and host cells that comprise a sequence which encodes a heavy chain and / or a light chain of said antibodies, and to a method of preparing said antibodies. The anti-CCL20 antibodies of the invention can be used in therapeutic applications to treat, for example, inflammatory and autoimmune disorders and cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
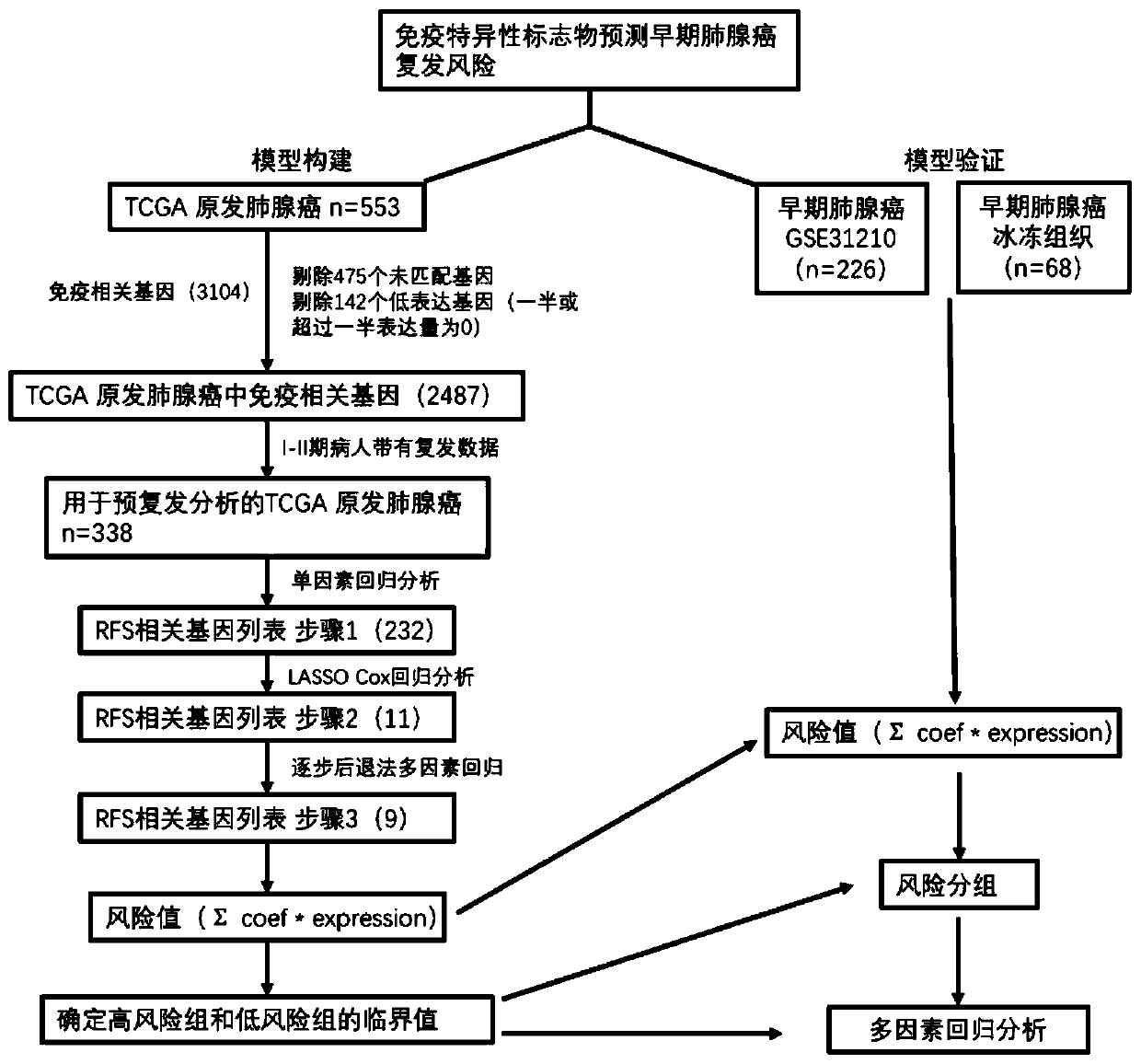
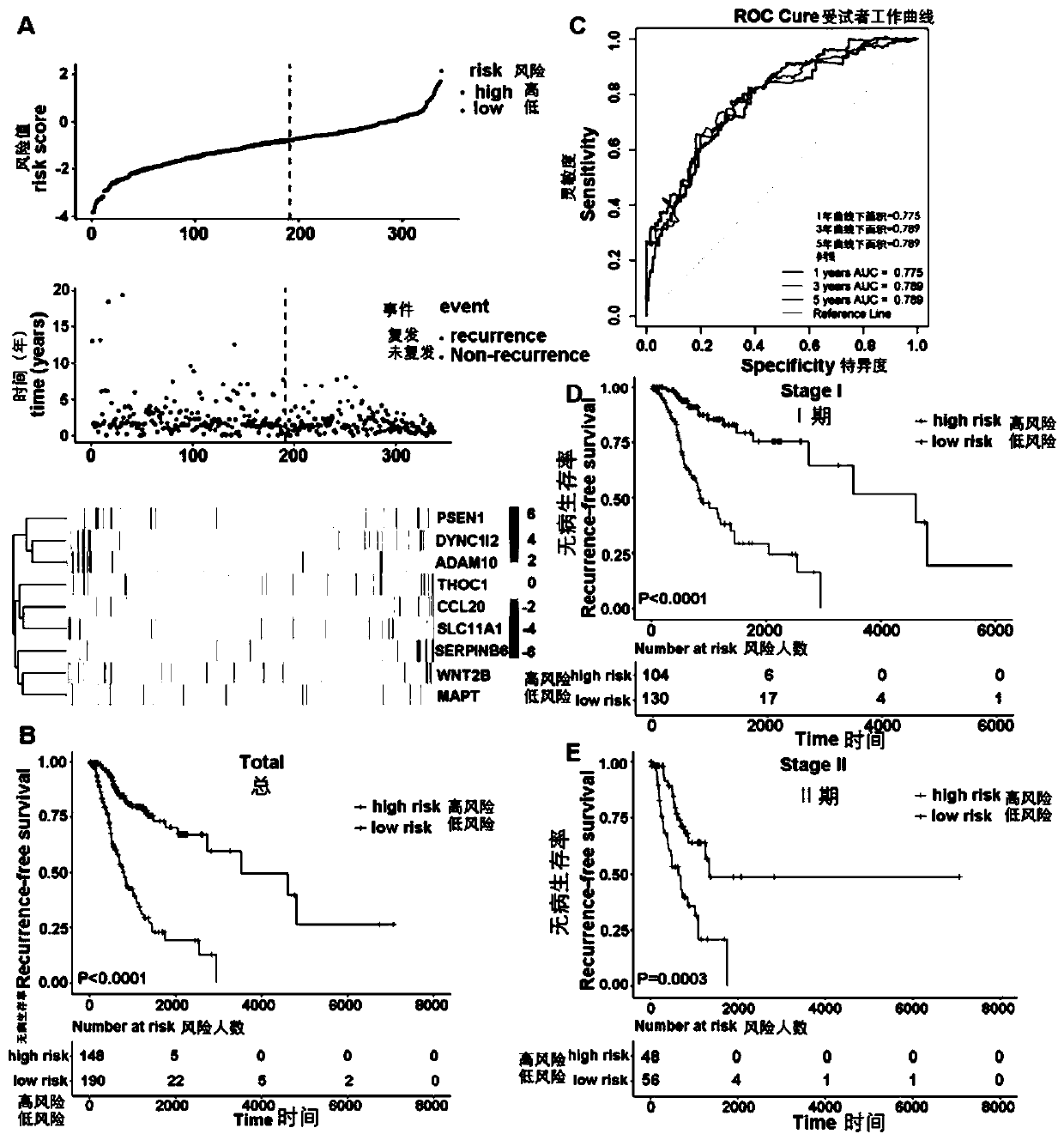
Prognosis evaluation system for patients with early-stage lung adenocarcinoma (LUAD) and application thereof
ActiveCN111394456AFacilitate understanding of the interaction mechanismMicrobiological testing/measurementProteomicsDiseasePulmonary adenocarcinoma
The invention discloses a prognosis evaluation system for patients with early-stage LUAD and application thereof. According to the invention, disease-free survival data of 632 patients with early-stage LUAD in three independent queues are integrated, and an individual IBRS model for patients with early-stage LUAD is established and verified. The three independent queues include TCGA, GSE31210 and68 cases of frozen tissues. The IBRS model is composed of the following nine genes: DYNC1I2, THOC1, ADAM10, SERPINB6, CCL20, WNT2B, SLC11A1, MAPT and PSEN1. The establishment of the IBRS model for patients with early-stage LUAD is not only beneficial for understanding of the complex interaction mechanism between immune molecules, but also brings great help to the recurrence prediction of the patients with early-stage LUAD and the optimization of a treatment scheme.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Ccl20-specific antibodies for cancer therapy
InactiveUS20110123542A1Inhibiting and reducing and growthInhibiting and reducing tumor progressionImmunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsTreatment fieldCancer therapy
The invention is directed to the field of cancer therapy, specifically to the use of anti-CCL20 antibodies for the treatment of neoplastic disorders. The invention provides compositions and methods useful for the treatment of CCR6 and CX-CR4 expressing tumors.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
Application of microRNA-210 inhibitor in preparing medicine treating inflammatory dermatosis
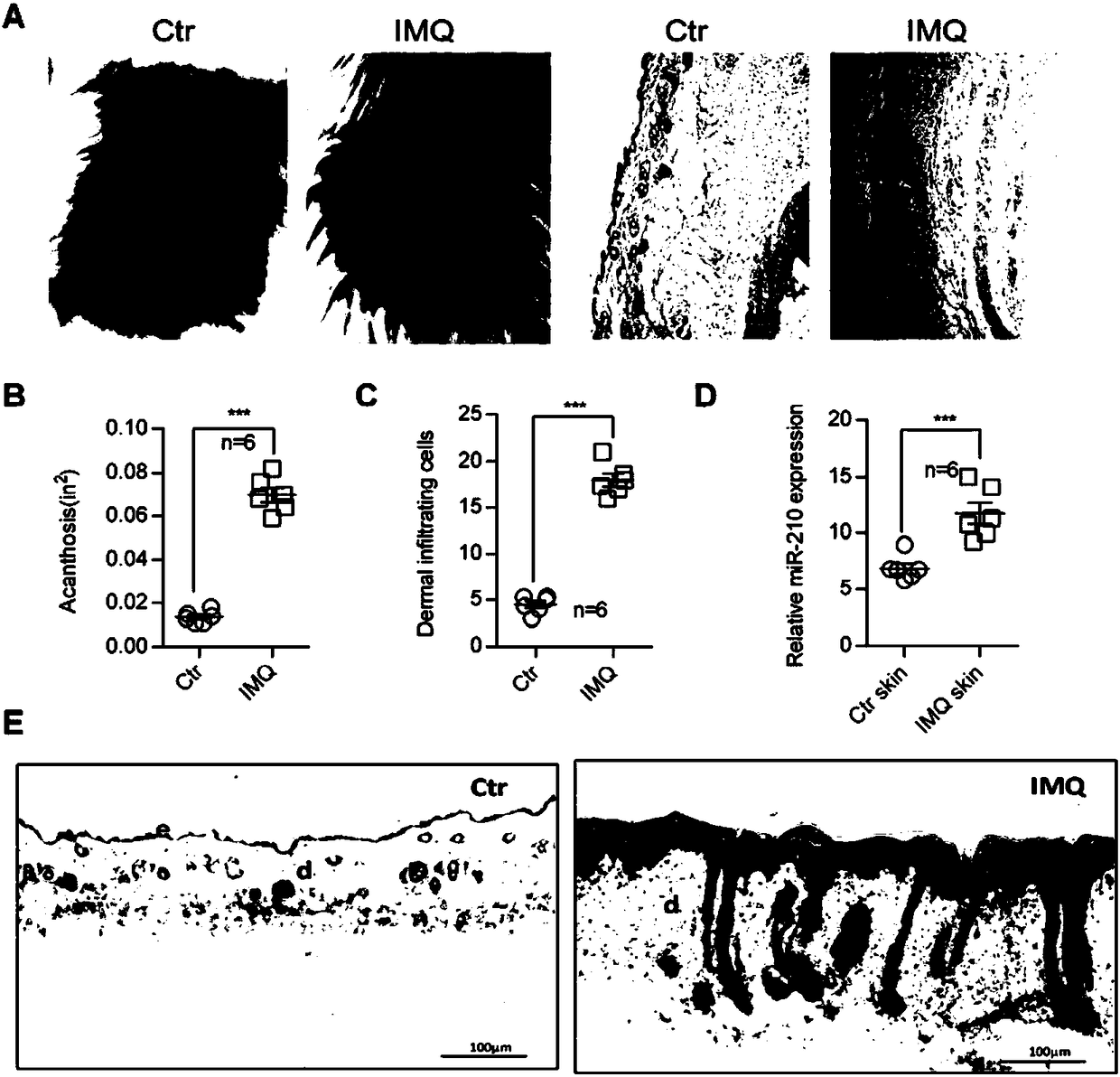
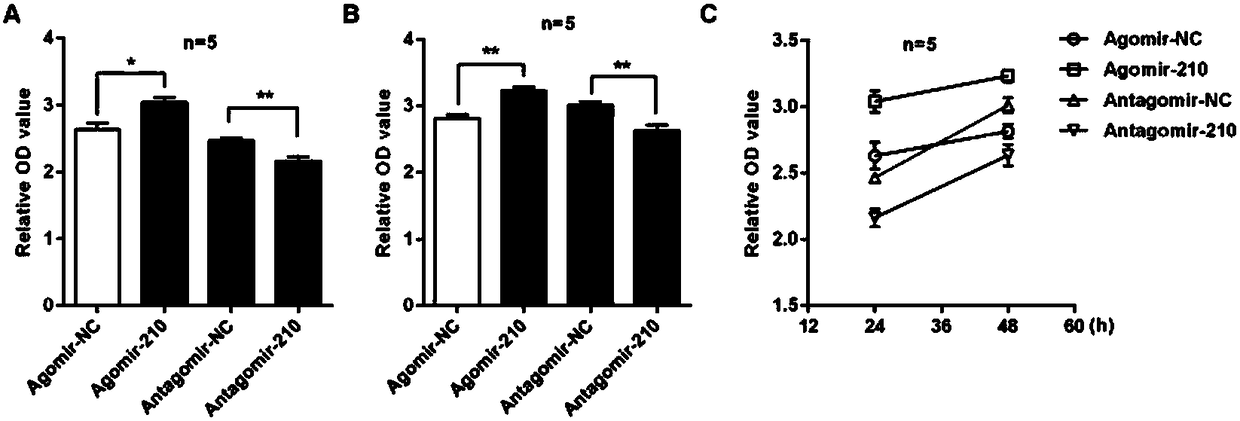
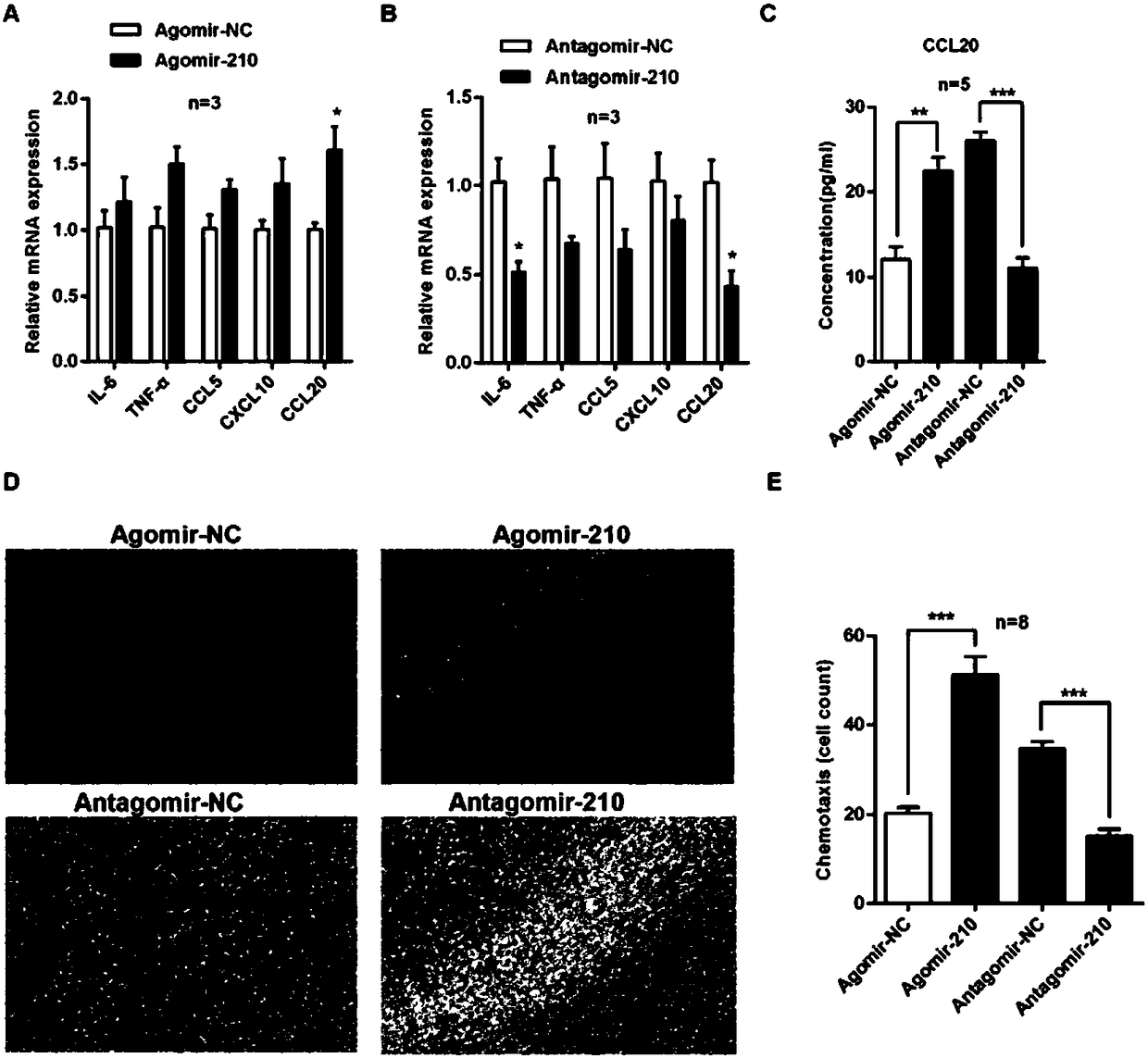
ActiveCN108144061AImprove inflammationPromote infiltrationOrganic active ingredientsPolymorphism usesCholesterolInflammatory dermatosis
The invention discloses application of microRNA-210 inhibitor in preparing medicine treating inflammatory dermatosis. A large quantity of experiments prove that in-vitro inhibiting of expression of microRNA-210 can obviously improve the expression of a target gene STAT6 of microRNA-210, thus the formation of cell proliferation by cutin and secretion of chemokine CCL20 are inhibited, migration of chemotaxis T cells of chemokine CCL20 to the skin lesion parts is further inhibited, and meanwhile, differentiation of TH1 and TH17 can be inhibited. MicroRNA-210 knockout and injection of the microRNA-210 inhibitor (antagomiR-210 modified by cholesterol) given into the skin lesion specifically inhibits the expression of microRNA-210, which can both obviously inhibit mouse skin inflammation and improve T cell immune imbalance. A novel pathophysiology mechanism is provided for inflammatory dermatosis, and a new strategy capable of being used for preparing the medicine treating inflammatory dermatosis is provided.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Endometrioid adenocarcinoma prognosis-related gene and protein as well as application thereof
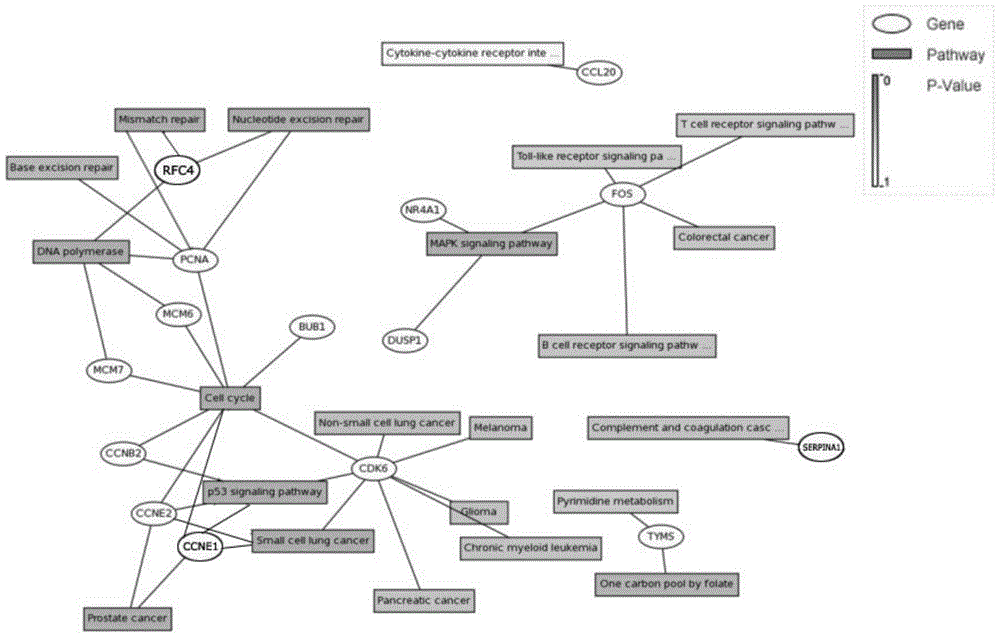
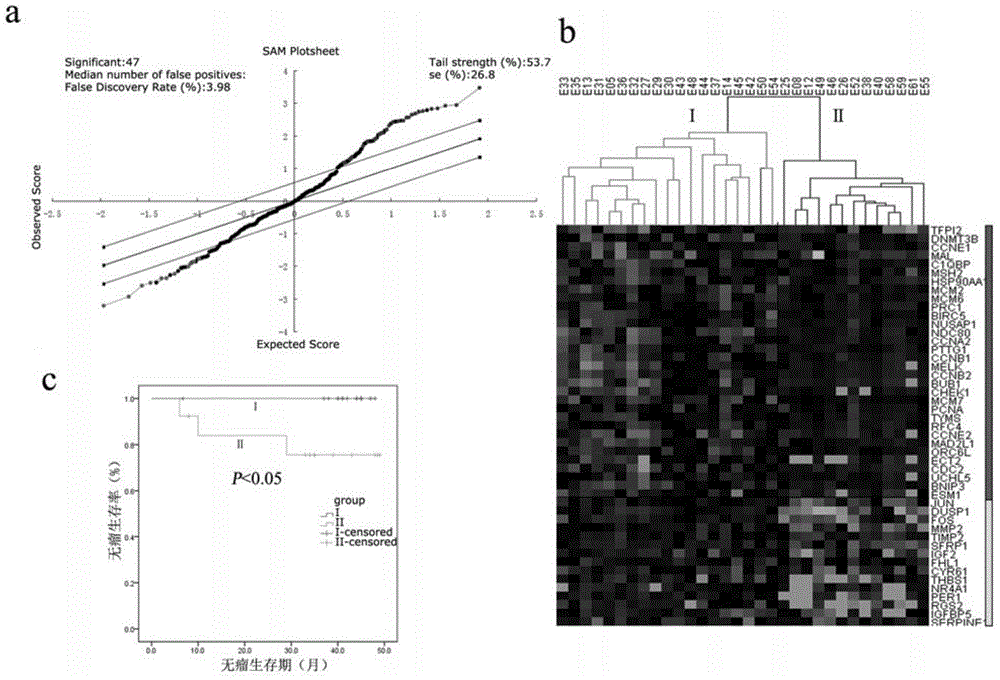
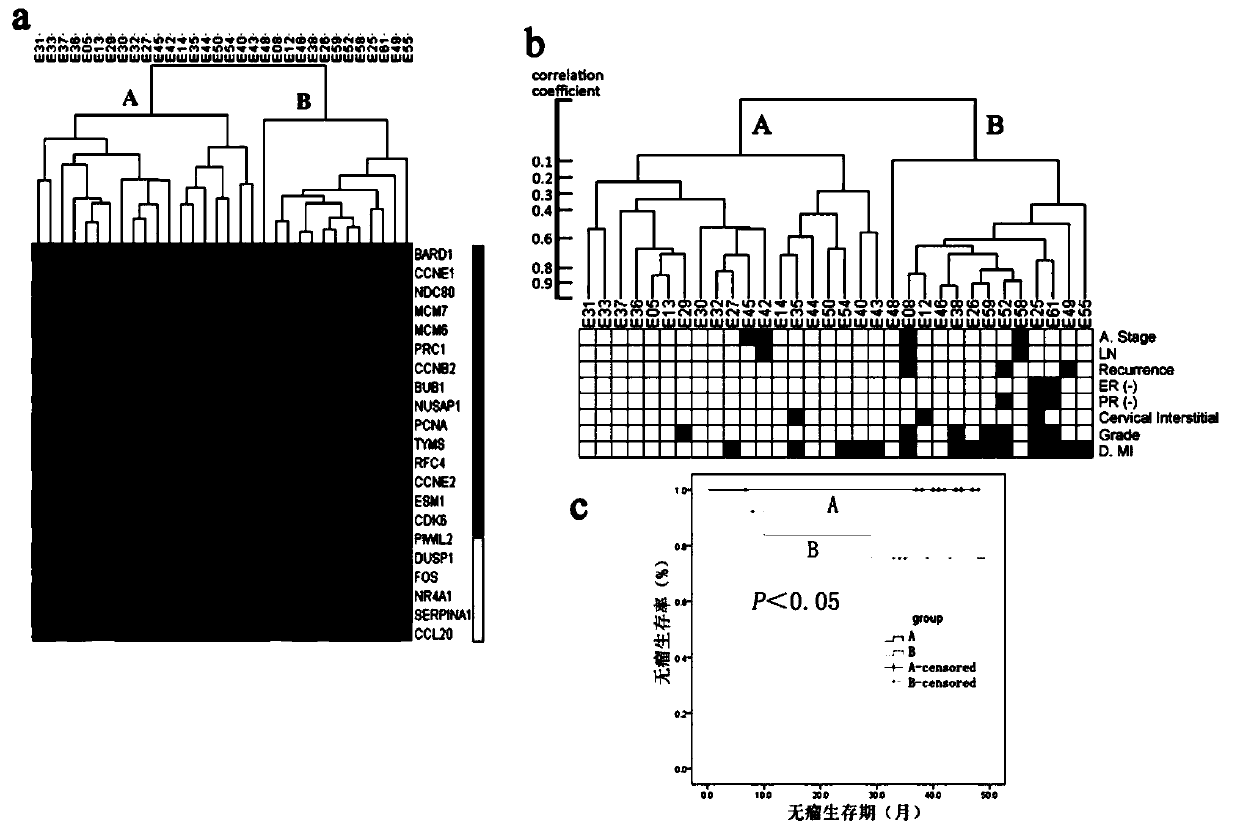
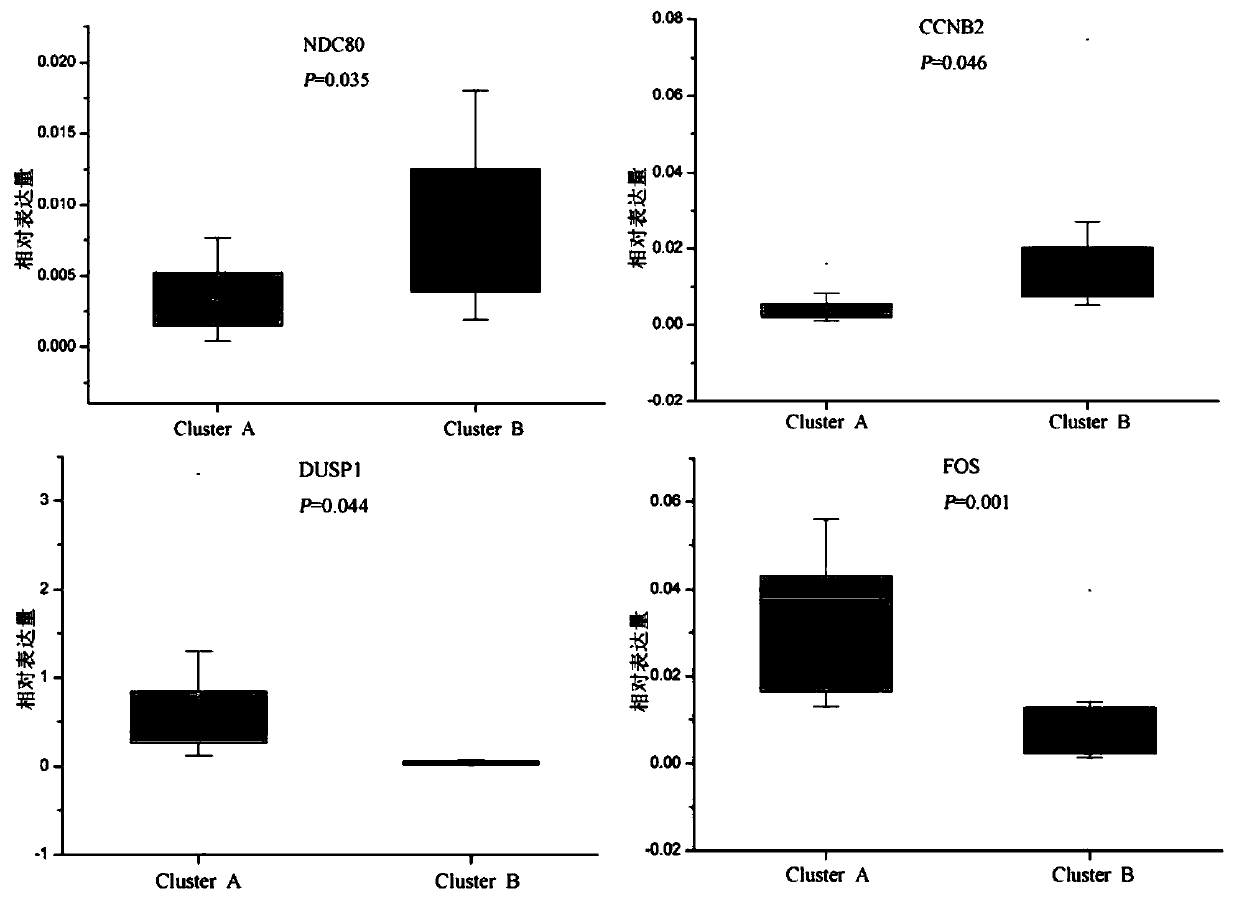
The invention discloses endometrioid adenocarcinoma prognosis-related gene and protein as well as an application thereof. The invention provides a kit a to assist grouping of patients population with endometrioid adenocarcinoma, comprising a substance for detecting mRNA level expression of 21 genes: PRC1, NUSAP1, MCM6, PIWIL2, TYMS, CCNB2, ESM1, BUB1, BARD1, MCM7, CDK6, PCNA, NDC80, CCNE2, RFC4, CCNE1, SERPINA1, FOS, DUSP1, CCL20, and NR4A1. The experiment found a gene model and a protein marker capable of performing prognostic prediction of disease-free survival time of endometrioid adenocarcinoma. The model has important clinical meaning for reflecting biological characteristics of endometrioid adenocarcinoma and prognostic prediction.
Owner:PEOPLES HOSPITAL PEKING UNIV
Targeting the histone code as a bacterial strategy for selectively modulating gene expression
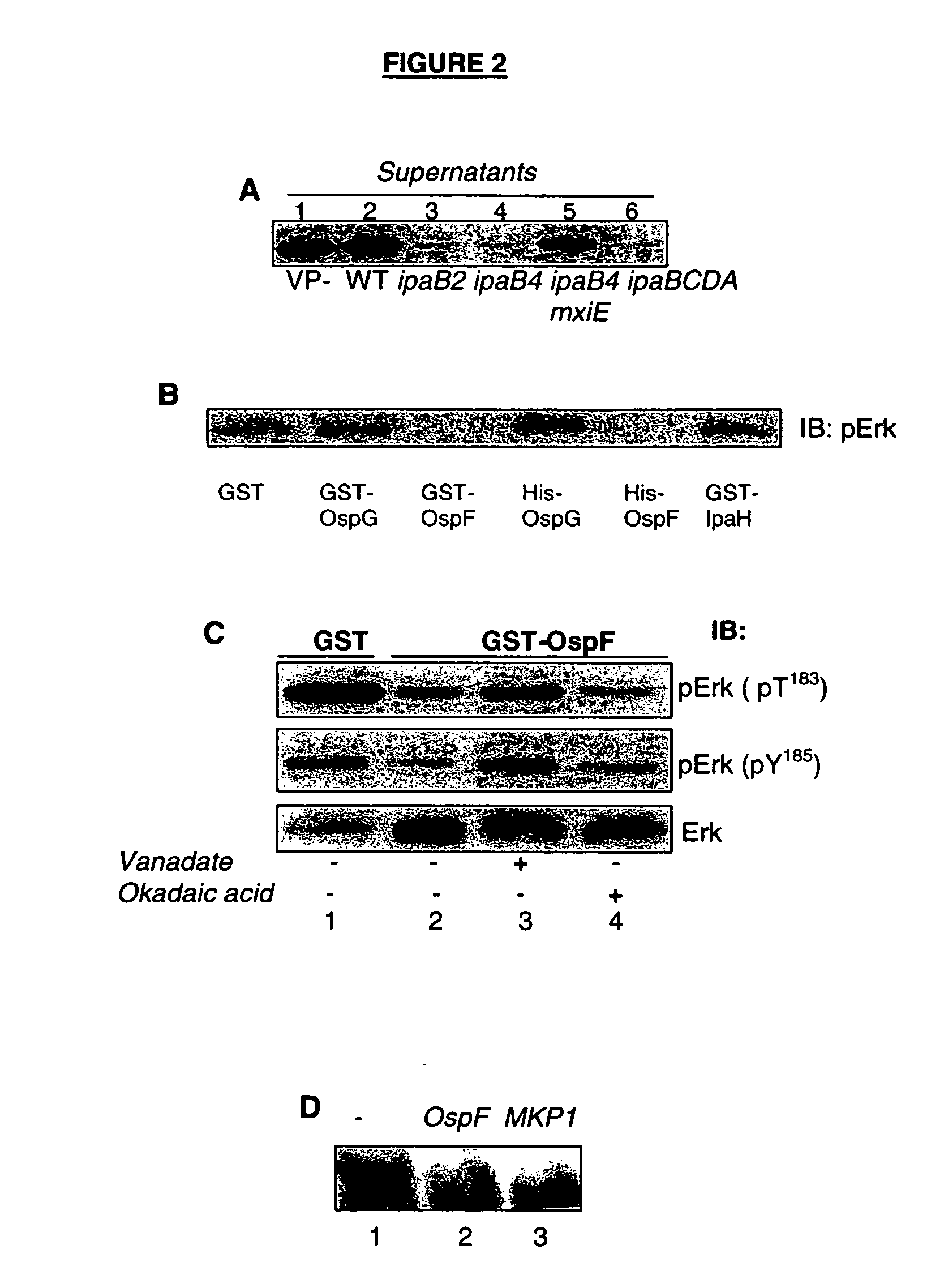
InactiveUS20070077254A1Reduce transcriptionAvoid changeAntibacterial agentsHydrolasesDiseaseShigella flexneri
The ospF gene of Shigella flexneri encodes a phosphatase, which is a member of a new class of phosphatases. The OspF phosphatase inhibits the activity of several proteins either by direct protein modification or transcription downregulation. These proteins include MAP kinase, IL-8, CCL20, IL-12, AP1, CREB, RPA p32, and BCL2 related proteins. Methods for treating diseases using OspF phosphatase, methods for identifying agents that modulate OspF phosphatase's activity, methods for identifying agents that mimic OspF phosphatase's activity, and immunogenic compositions comprising OspF phosphatase are provided. A strain of Shigella flexneri containing an inactivated ospF gene is also provided.
Owner:INST PASTEUR
Quick quantitative detection method and kit for human copeptin
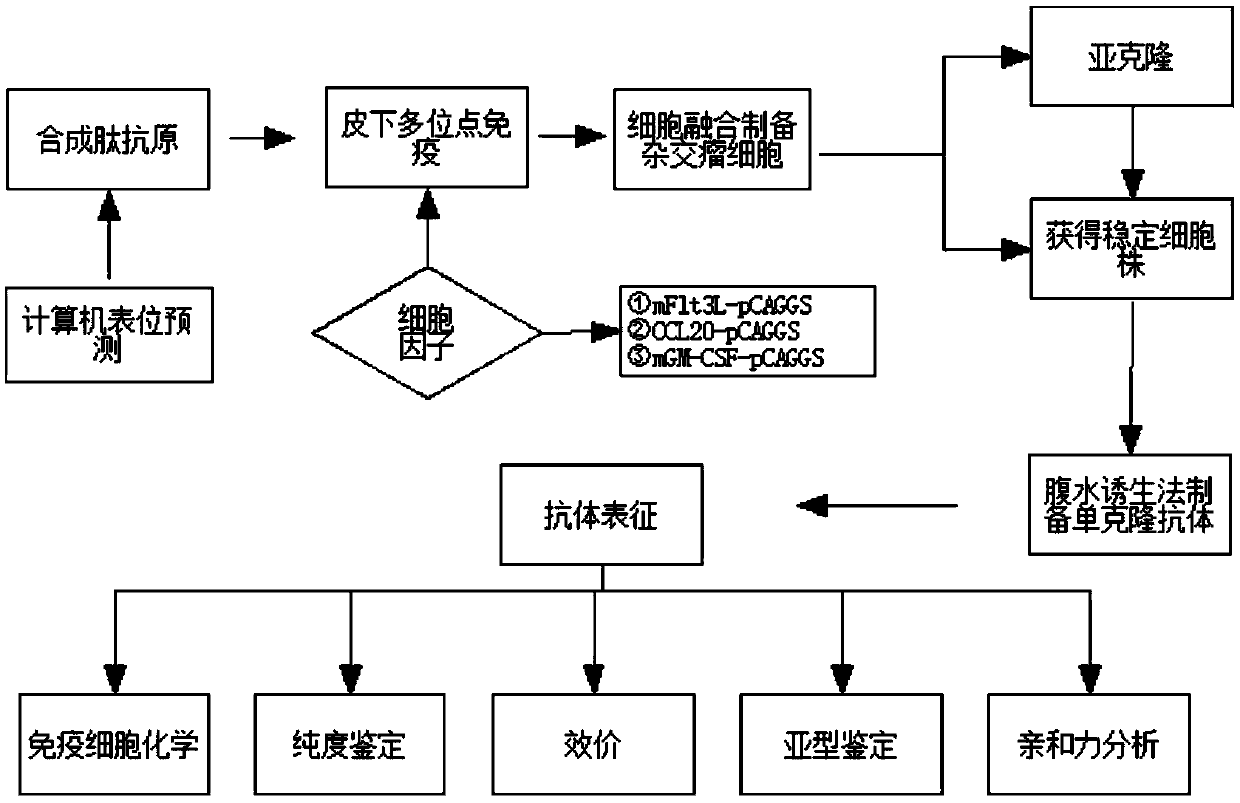
InactiveCN109596839AEasy to adjustHigh clinical application valueBiological testingSerum igeMagnetic bead
The invention provides a method for detecting CPP (Copeptin) by a full-automatic chemiLuminescence method on the basis of MPs (magnetic particles). Common immunity and immunoregulation molecules are combined, cell factors Flt3l (Mouse fms-like tyrosinekinase 3ligand), mGM-CSF (Mouse Granulocyte Macrophage Colony Stimulating Factor) and CCL20 (Mouse CC subtype chemokine ligand 20) are assisted in delivering while conventional common immunity is carried out to obtain an antibody with high affinity, the antibody is combined with magnetic separation, a magnetic bead has a large superficial area and a large area ratio, so that the CPP with the low concentration can be effectively enriched, and therefore, CPP in human serums can be quickly and sensitively measured. The measurement time, the sensitivity and the specificity of the CPP can be obviously improved.
Owner:GUANGZHOU WONDFO BIOTECH
Antagonistic human light-specific human monoclonal antibodies
ActiveCN101663321AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseMonoclonal antibody
Provided herein are antibodies, such as fully human antibodies, that immunospecifically bind to an hLIGHT polypeptide. Also provided are isolated nucleic acids encoding antibodies, such as fully humanantibodies, that immunospecifically bind to a hLIGHT polypeptide. Further provided are vectors and host cells comprising nucleic acids encoding antibodies, such as fully human antibodies, that immunospecifically bind to a hLIGHT polypeptide. Also provided are methods of making antibodies, such as fully human antibodies, that immunospecifically bind to a hLIGHT polypeptide. Also provided herein isa method of treating a hLIGHT- mediated disease in a subject comprising administering to the subject an antibody, such as a fully human antibody, that immunospecifically binds to a hLIGHT polypeptide. In preferred embodiments, that anti-hLIGHT antibodies provided herein will ameliorate, neutralize or otherwise inhibit hLIGHT biological activity in vivo (e.g., the hLIGHT- mediated production or secretion of CCL20, IL-8 or RANTES from a cell expressing a hLIGHT receptor). Also provided herein is a method for the detection of hLIGHT in a sample as well as a method for ameliorating, neutralizingor otherwise inhibiting hLIGHT activity, e.g., in a human subject suffering from a disorder in which hLIGHT activity is detrimental.
Owner:KYOWA HAKKO KIRIN CO LTD +1
Methods for detection of respiratory diseases
ActiveUS9952225B2Disease diagnosisBiological testingObstructive Pulmonary DiseasesPulmonary circulation diseases
Owner:NAT JEWISH HEALTH
Methods and compositions for the treatment and prevention of cancer
ActiveUS8258278B2Tumor rejection antigen precursorsAntibody mimetics/scaffoldsCancer preventionOncology
Owner:UNITED STATES OF AMERICA
Method used for evaluating immunomodulatory effect of lactobacillus acidophilus and application of method
InactiveCN106480190AOvercome the deficiency of immunomodulatory function evaluationEffective evaluationMicrobiological testing/measurementMaterial analysisCXCR4CCL2
The invention discloses a method used for evaluating an immunomodulatory effect of lactobacillus acidophilus and an application of the method, and belongs to the field of biotechnology. According to the method provided by the invention, a dynamic change situation of immunity-related differentially expressed genes is detected, wherein the differentially expressed genes include up-regulated immunity-related genes CCL2, CCL20, IL1 alpha, IL1 beta, IL6ST, IL8, PTX3 and TNFRSF9 and a down-regulated immunity-related gene CXCR4. Through the method, the immunomodulatory effect of the lactobacillus acidophilus can be evaluated only by detecting nine immunity-related genes, and quick detection can be realized by utilizing Real Time PCR, so that the time is greatly shortened and the deficiency of existing evaluation of the immunomodulatory effect of the lactobacillus acidophilus is overcome; and the method is well verified in a mouse test and a Caco-2 cell.
Owner:黑龙江省绿色食品科学研究院
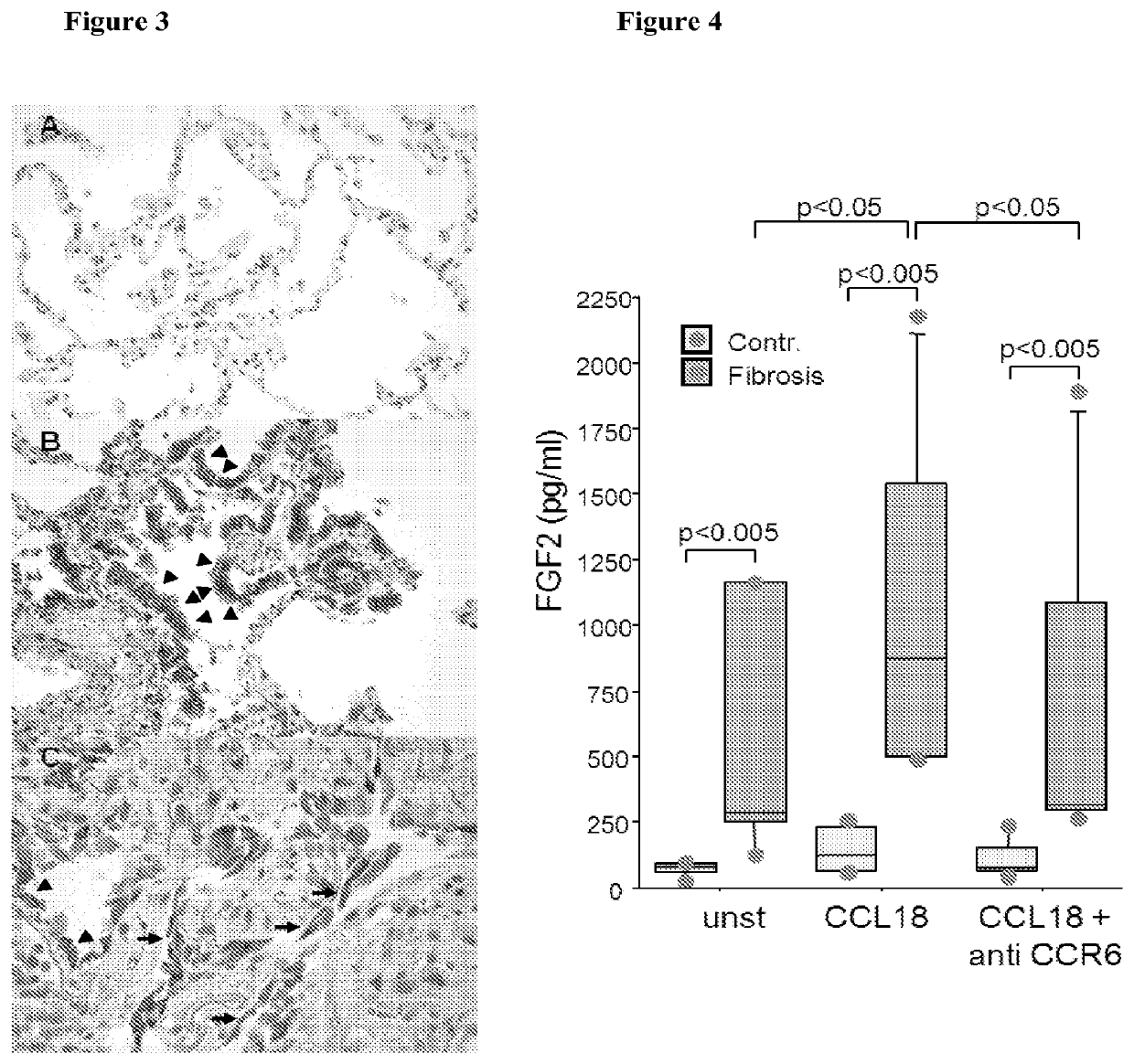
Blockade of ccl18 signaling via ccr6 as a therapeutic option in fibrotic diseases and cancer
InactiveUS20130109629A1Increase percentageCompound screeningApoptosis detectionDiseaseInterstitial lung disease
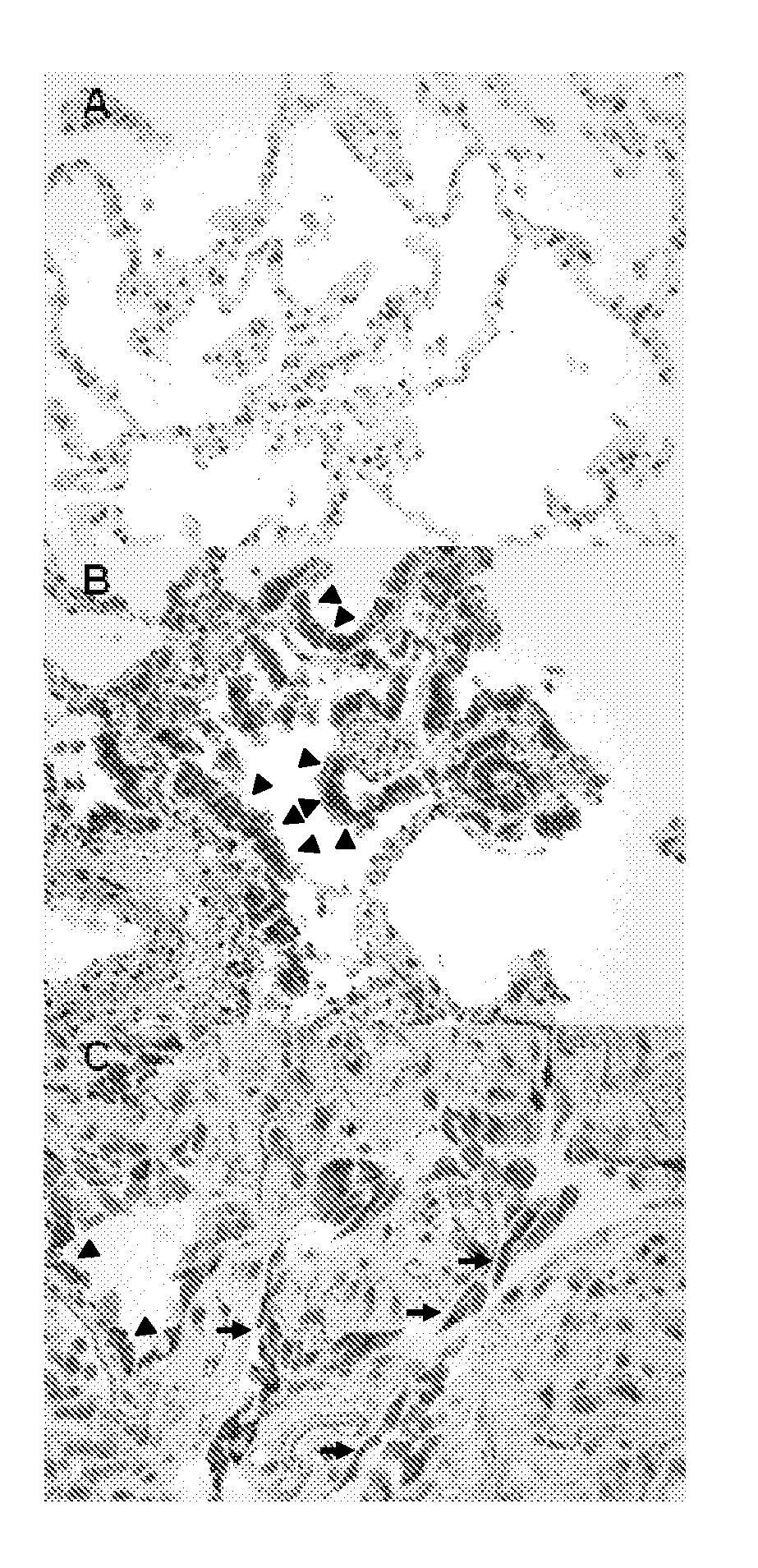
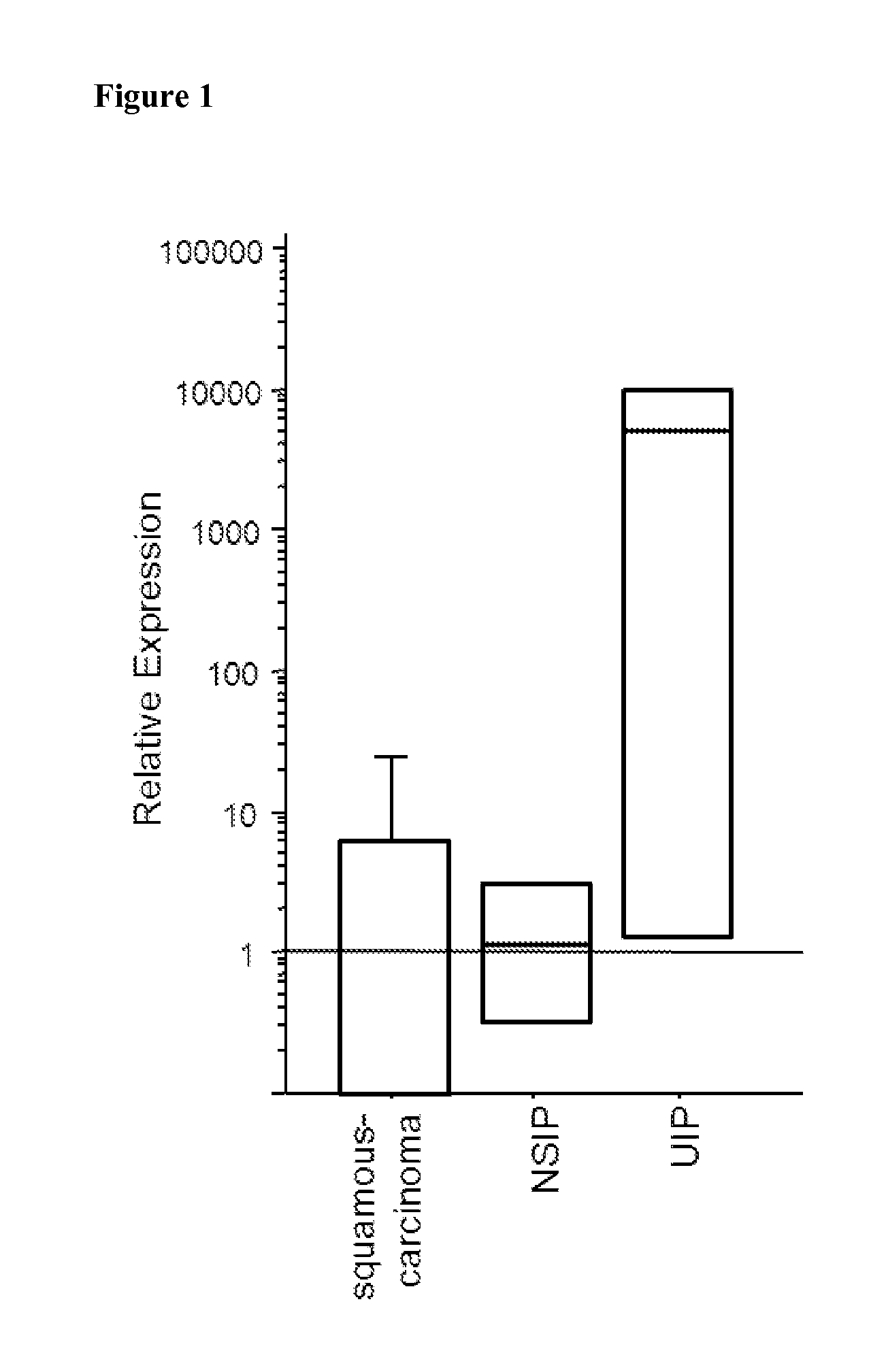
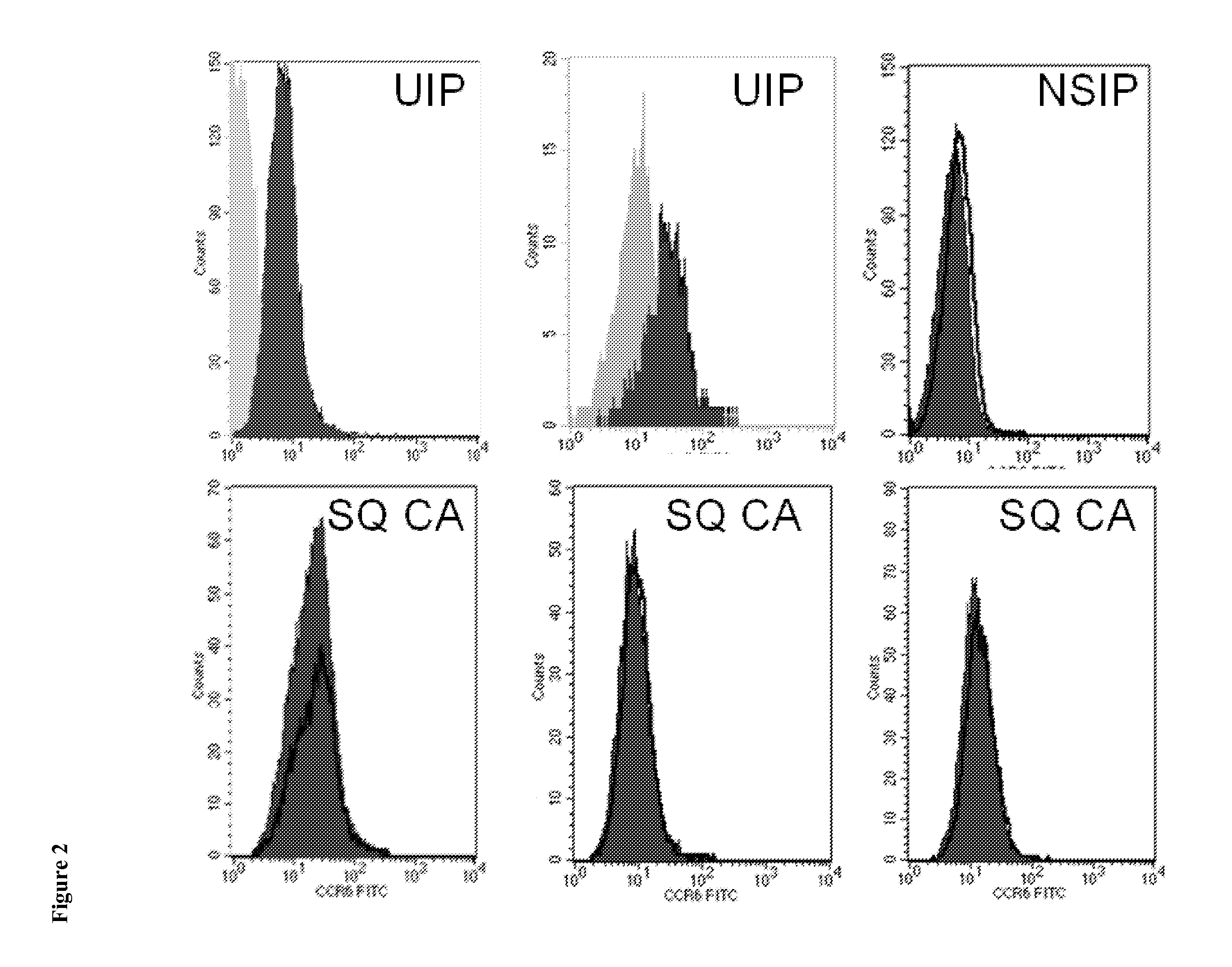
The present invention relates to an isolated soluble CCR6 receptor polypeptide capable of binding to CCL18 and / or CCL20 and to a method for quantifying the concentration of a soluble CCR6 receptor polypeptide in a liquid sample from a subject. The present invention also relates to a method for detecting and / or prognosticating an interstitial lung disease or a cancer in a subject by determining the level of a soluble CCR6 receptor polypeptide in a sample from said subject and further provides a pharmaceutical composition comprising a compound capable of inhibiting the activity and / or the expression of CCL18 or CCL20 for the treatment of said diseases. The present invention further relates to an isolated polypeptide capable of binding to and inhibiting the activity of the chemokine receptor CCR6 and to a method for identifying further inhibitors of CCR6 receptor activity. The present invention also relates to a method for detecting an interstitial lung disease or a cancer in a subject by determining the level of CCR6 gene expression in a sample from said subject and further provides pharmaceutical compositions comprising inhibitors of CCR6 receptor activity and / or expression for the treatment of said diseases.
Owner:UNIVERSITATSKLINIKUM FREIBURG
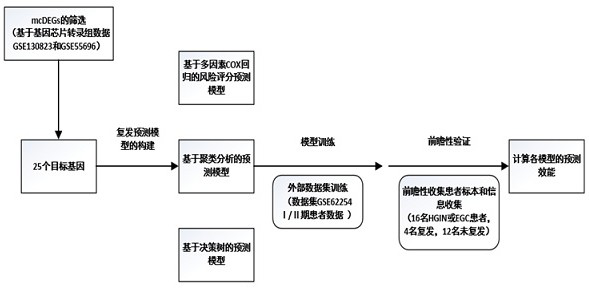
Early gastric cancer prognosis differential gene and recurrence prediction model
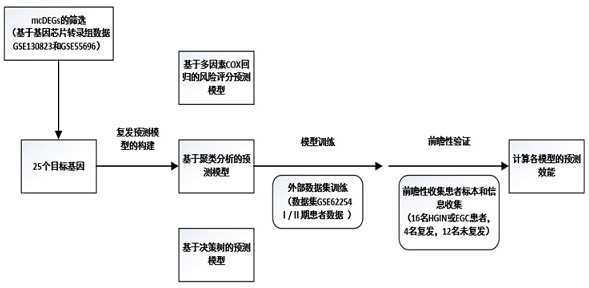
ActiveCN114107515AIncreased sensitivityAdjust the frequency of follow-up visitsMicrobiological testing/measurementDNA/RNA fragmentationRecurrence predictionGastric carcinoma
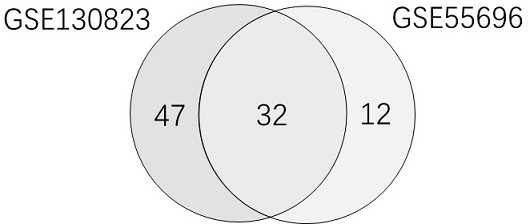
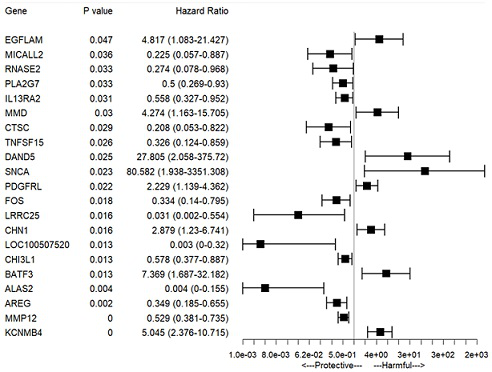
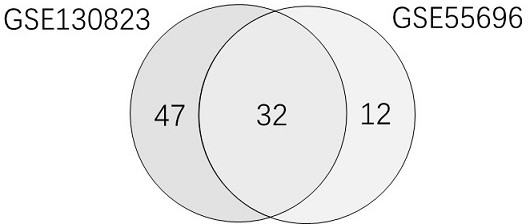
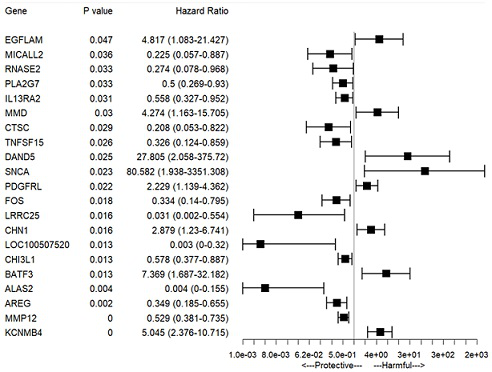
The invention relates to establishment of an early gastric cancer recurrence prediction model, 25 potential genes related to early gastric cancer recurrence are screened out by using two batches of gene chip transcriptome data of GSE130823 and GSE55696, and the recurrence prediction model based on eight genes of AREG, LOC100507520, MMD, CH3L1, FOS, CCL20, CXCR2 and BATF3 is established. The model has excellent sensitivity, that is, all patients which are predicted to be not relapsed do not relapse, and the clinical prompting significance is that the reexamination follow-up diagnosis frequency of the patients can be adjusted according to the reexamination follow-up diagnosis frequency.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Blockade of CCL18 signaling via CCR6 as a therapeutic option in treating interstitial lung disease
The present invention relates to an isolated soluble CCR6 receptor polypeptide capable of binding to CCL18 and / or CCL20 and to a method for quantifying the concentration of a soluble CCR6 receptor polypeptide in a liquid sample from a subject. The present invention also relates to a method for detecting and / or prognosticating an interstitial lung disease or a cancer in a subject by determining the level of a soluble CCR6 receptor polypeptide in a sample from said subject and further provides a pharmaceutical composition comprising a compound capable of inhibiting the activity and / or the expression of CCL18 or CCL20 for the treatment of said diseases. The present invention further relates to an isolated polypeptide capable of binding to and inhibiting the activity of the chemokine receptor CCR6 and to a method for identifying further inhibitors of CCR6 receptor activity. The present invention also relates to a method for detecting an interstitial lung disease or a cancer in a subject by determining the level of CCR6 gene expression in a sample from said subject and further provides pharmaceutical compositions comprising inhibitors of CCR6 receptor activity and / or expression for the treatment of said diseases.
Owner:UNIVERSITATSKLINIKUM FREIBURG
Construction method of animal and cell models for improving rosacea inflammation by artemisinin
InactiveCN111925979ASymptoms improvedImprove skin erythemaEpidermal cells/skin cellsArtificial cell constructsDiseaseCCL2
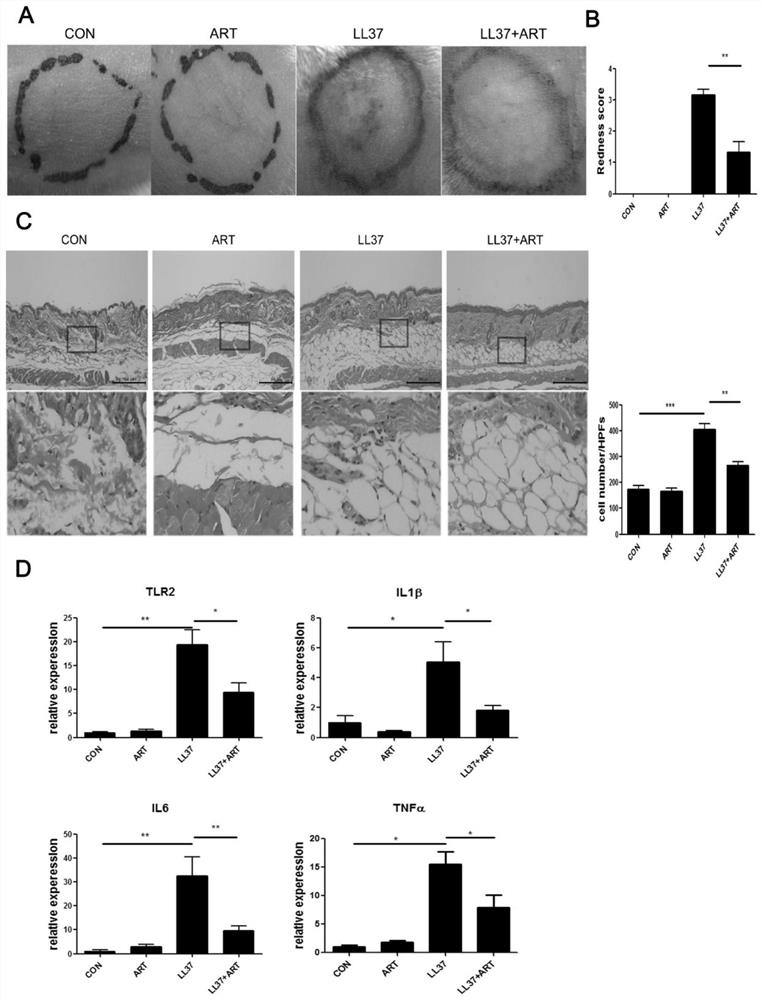
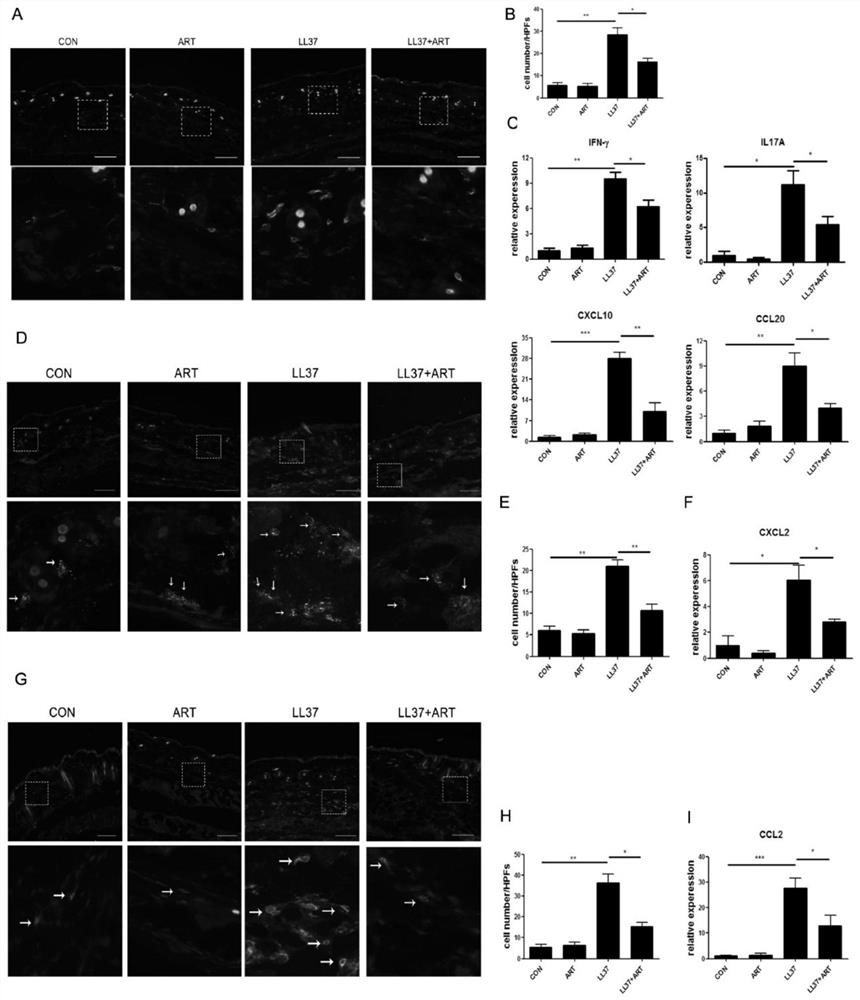
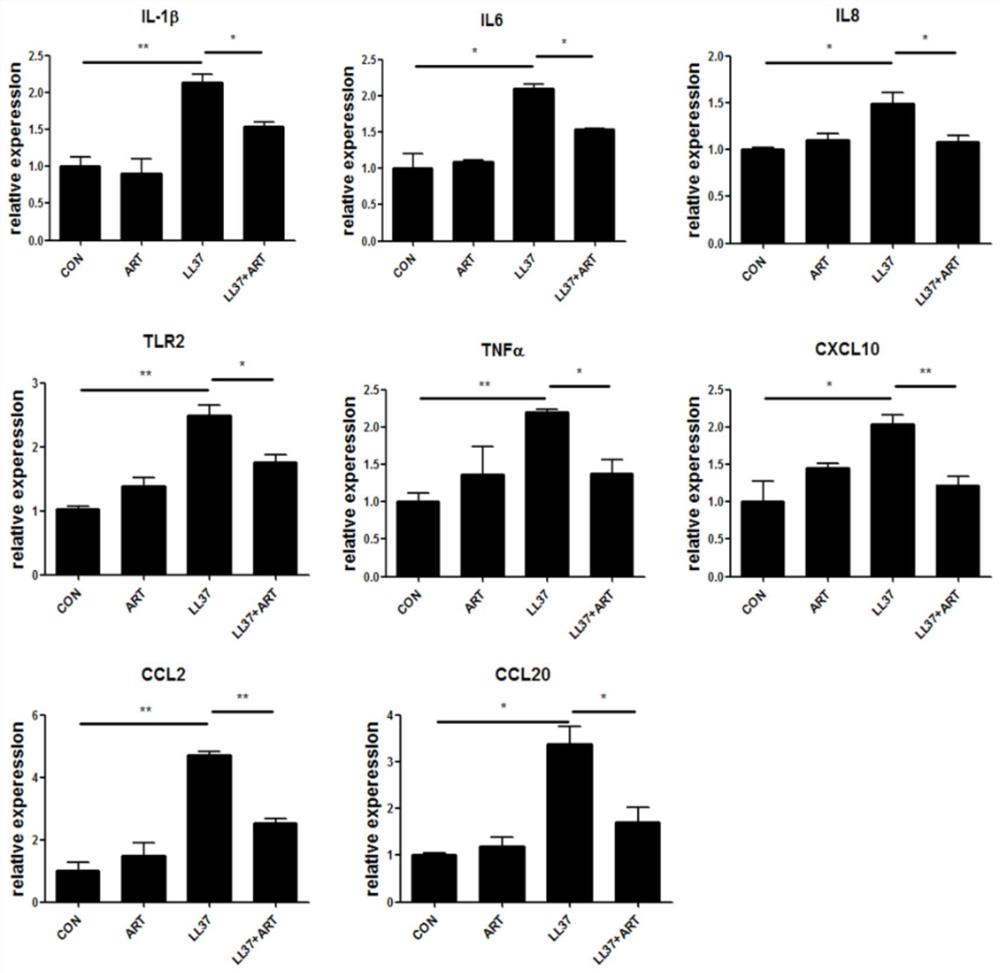
The invention discloses a construction method of animal and cell models for improving rosacea inflammation by artemisinin, and relates to the technical field of skin disease medicaments, in particularto a construction method of animal and cell models for improving rosacea inflammation by artemisinin. The method comprises the following steps of: (1) constructing an animal model for improving rosacea inflammation phenotype by artemisinin; and (2) constructing a cell model for improving rosacea inflammatory phenotype by artemisinin. The invention has the advantages that a potential new drug (artemisinin) for treating rosacea is discovered; the pathogenesis of the rosacea is explored; it is found that artemisinin can obviously improve LL37-induced rosacea-like inflammatory mouse skin lesion erythema, histopathological changes and increase of IL-1 beta, IL-6, TNF alpha and TLR2; meanwhile, artemisinin can reduce infiltration of skin lesion CD4 + T cells, macrophages and neutrophils and inhibit expression of immune cell related chemokines (CXCL10, CCL20, CCL2 and CXCL2) in skin lesion.
Owner:GUIZHOU PROVINCIAL PEOPLES HOSPITAL
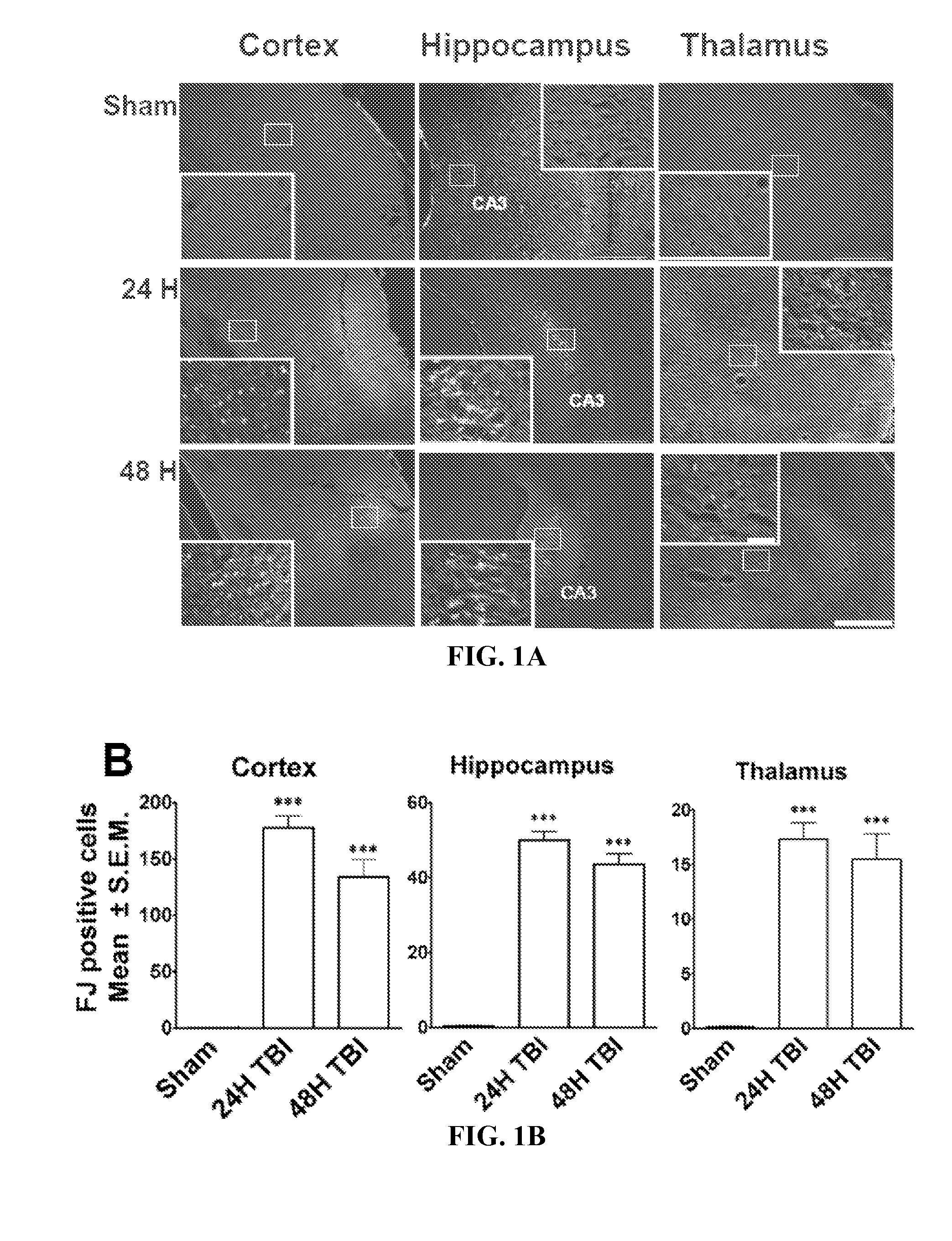
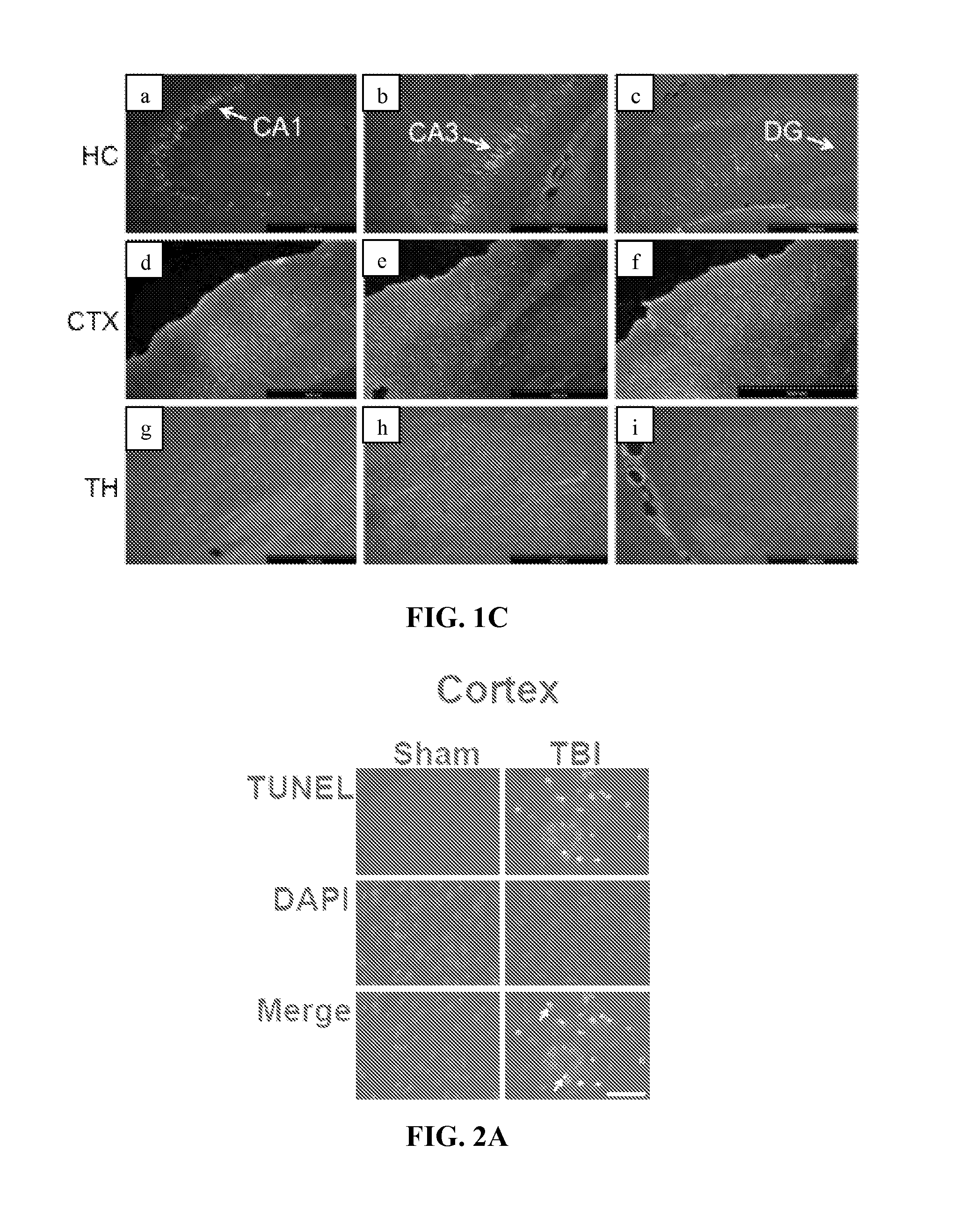
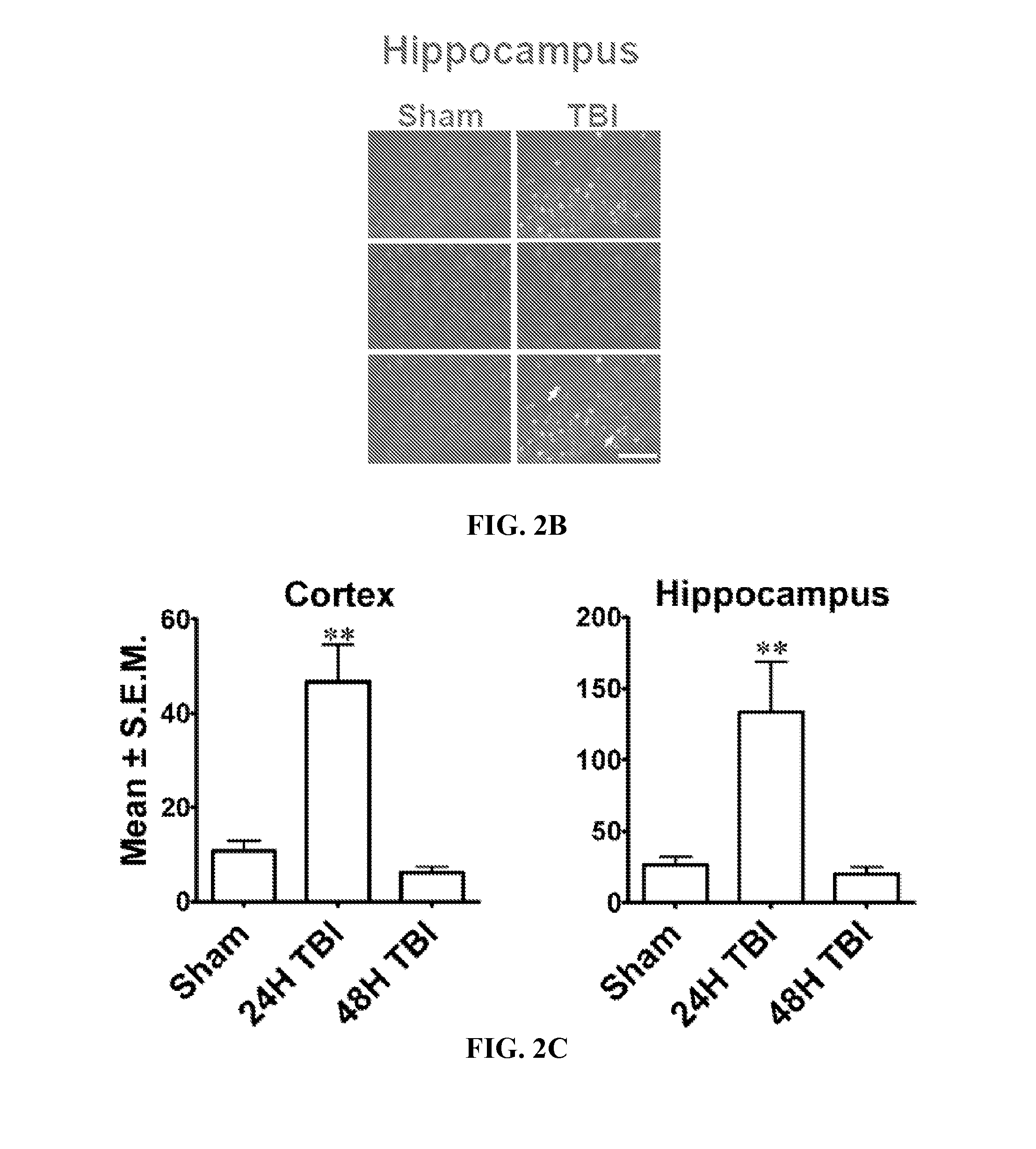
Diagnosis and treatment of traumatic brain injury
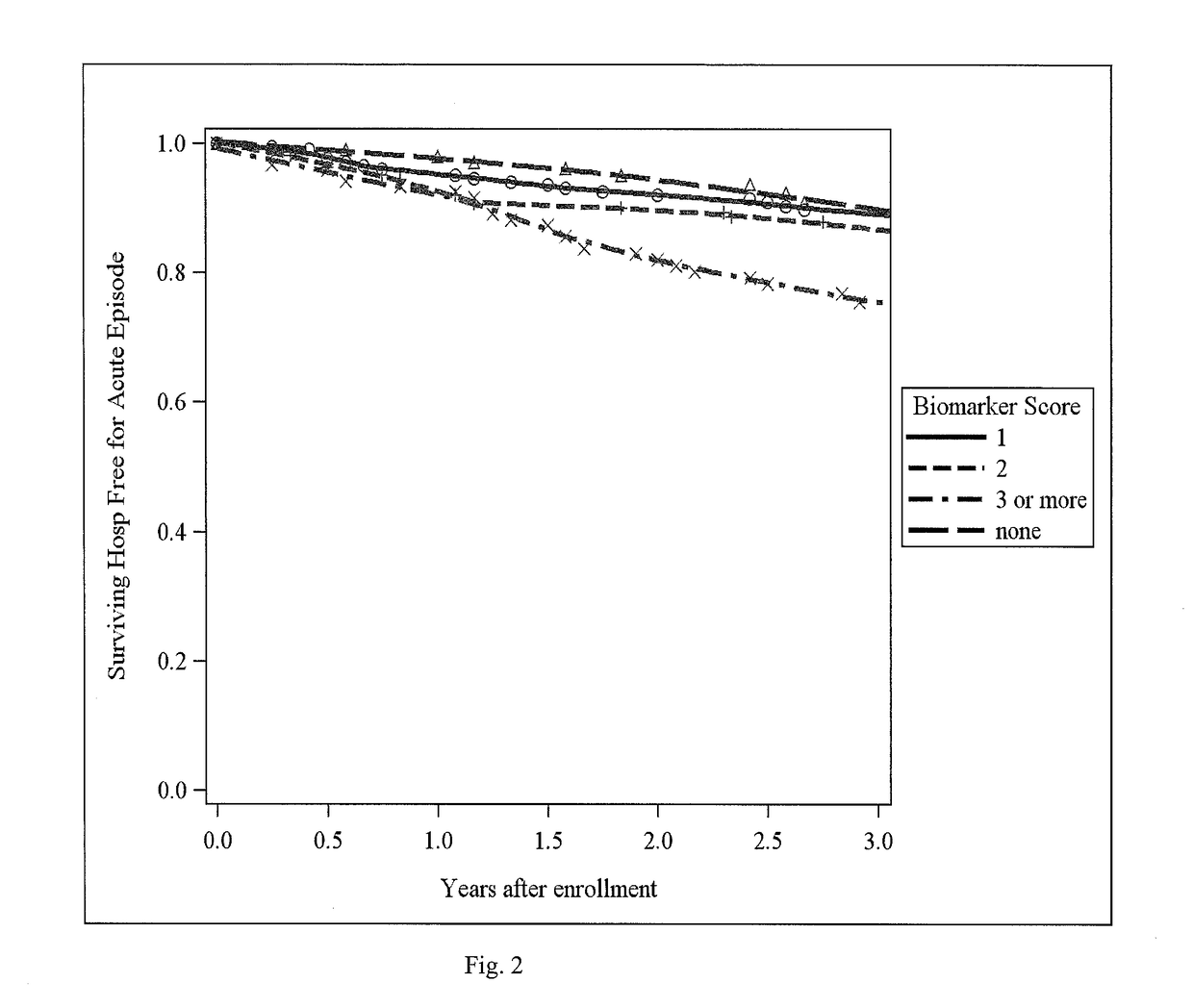
The subject invention identifies CC chemokine ligand 20 (CCL20) as a novel biomarker for diagnosis of traumatic brain injury and / or neurodegeneration in the brain. The subject invention also provides treatment methods for traumatic brain injury and / or neurodegeneration in the brain by modulating systemic and / or brain-specific CCL20-CCR6 signaling. Also provided are uses of CCL20-CCR6 signaling a target for screening for therapeutic agents that are useful for treatment of traumatic brain injury.
Owner:UNIV OF SOUTH FLORIDA
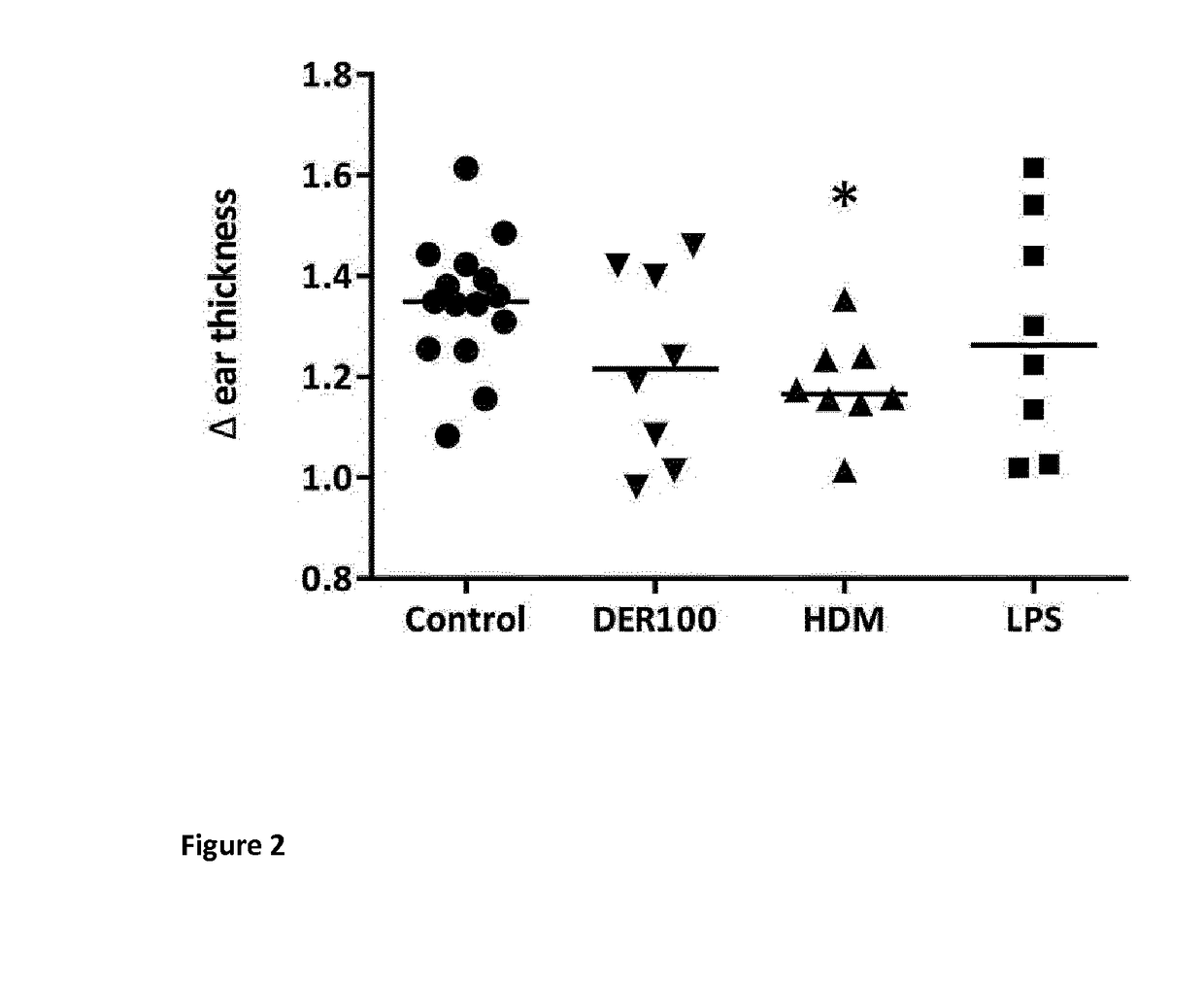
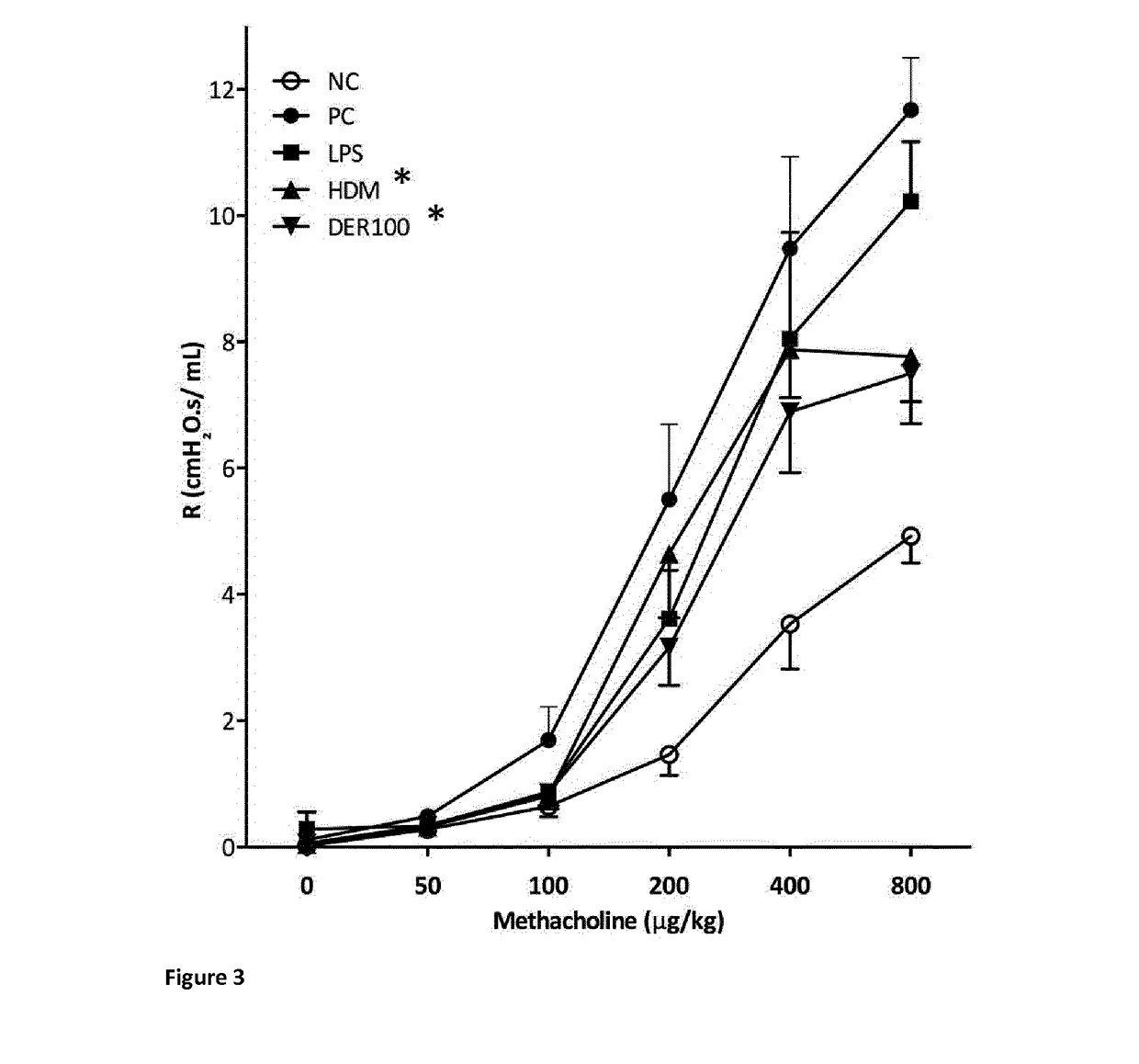
Allergy-specific immunotherapy compositions for use in the treatment of house-dust mite allergy
InactiveUS20180169220A1Effective in eliciting desensitizationRelieve symptomsAntipyreticAnalgesicsAfter treatmentCell activity
The present invention relates to a composition comprising purified natural Der p1 and purified natural Der p2 allergens for use in the treatment of house-dust mite allergy in mammals, like humans. The invention further pertains pharmaceutical compositions for allergy-specific immune therapy. Specifically, the invention discloses reduced responsiveness to subsequent house dust mite allergen exposure after treatment with a pharmaceutical composition comprising purified natural Der p1 and purified natural Der p2 characterized by reduction in Th2 cell activity and reduction in CCL20 release.
Owner:CITEQ
Immunoadjuvant of diffuse large B-cell lymphoma and application of immunoadjuvant
ActiveCN110812476AImprove anti-tumor effectReduced proliferation rateCancer antigen ingredientsAntineoplastic agentsIn vivoLocal injection
The invention discloses an immunoadjuvant based on diffuse large B-cell lymphoma of a chemotactic factor CCL20 and an application of the immunoadjuvant. Target ODN of a total-phosphorothioate chemotactic factor CCL20mRNA 3'UTR is used as the immunoadjuvant to be applied to the diffuse large B-cell lymphoma. The immunoadjuvant exerts favorable antitumor effects on DLBCL in vivo and in vitro research, and through co-hatching of the immunoadjuvant and DLBCL cells, the cell proliferation rate of the DLBCL can be obviously reduced; after the immunoadjuvant and inactivated tumor cells are mixed forlocal injection, the general information of mice is favorable, and the mice do not have abnormal conditions of convulsions, disturbance, pilo-erection, twitch and the like. The immunoadjuvant does nothave abnormal phenomena of red and swollen, inflammation, ulceration, scleroma and the like in local injection parts. The mice without bearing cancer do not have the death phenomenon, the immunoadjuvant is promoted to be a DLBCL immunoadjuvant, can be used for clinical treatment, and has wide prospects.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Compositions And Methods For Treating Cancer
InactiveUS20180237515A1Reducing progression rateReduce capacityBiological material analysisImmunoglobulins against cytokines/lymphokines/interferonsCCL20Cancer research
Owner:MEDIMMUNE LLC
Applications of TRAIL truncated mutant in activating NF-kappaB and in inflammatory responses
InactiveCN102908612AAffect the inflammatory responsePeptide/protein ingredientsAntipyreticApoptosisADAMTS Proteins
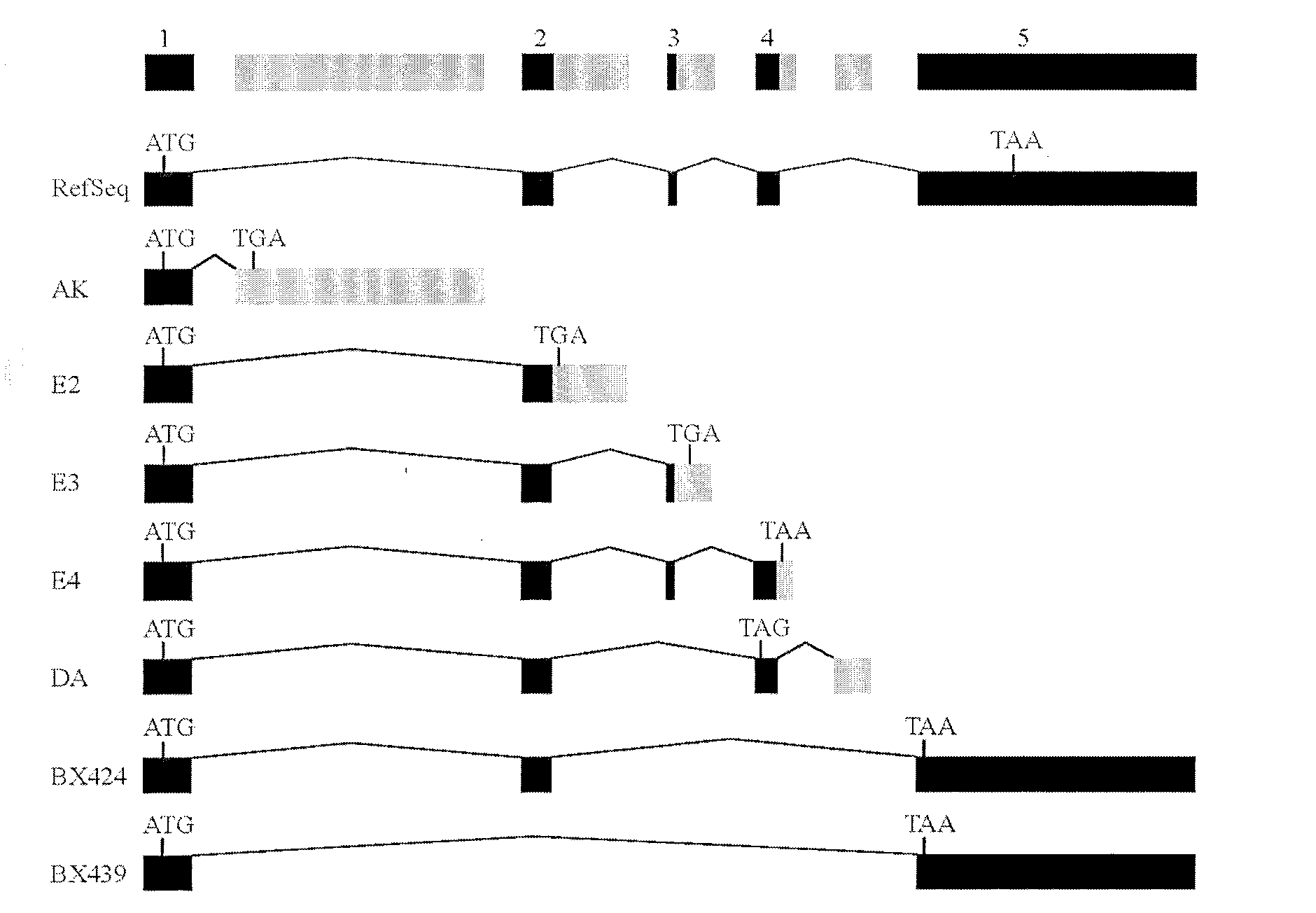
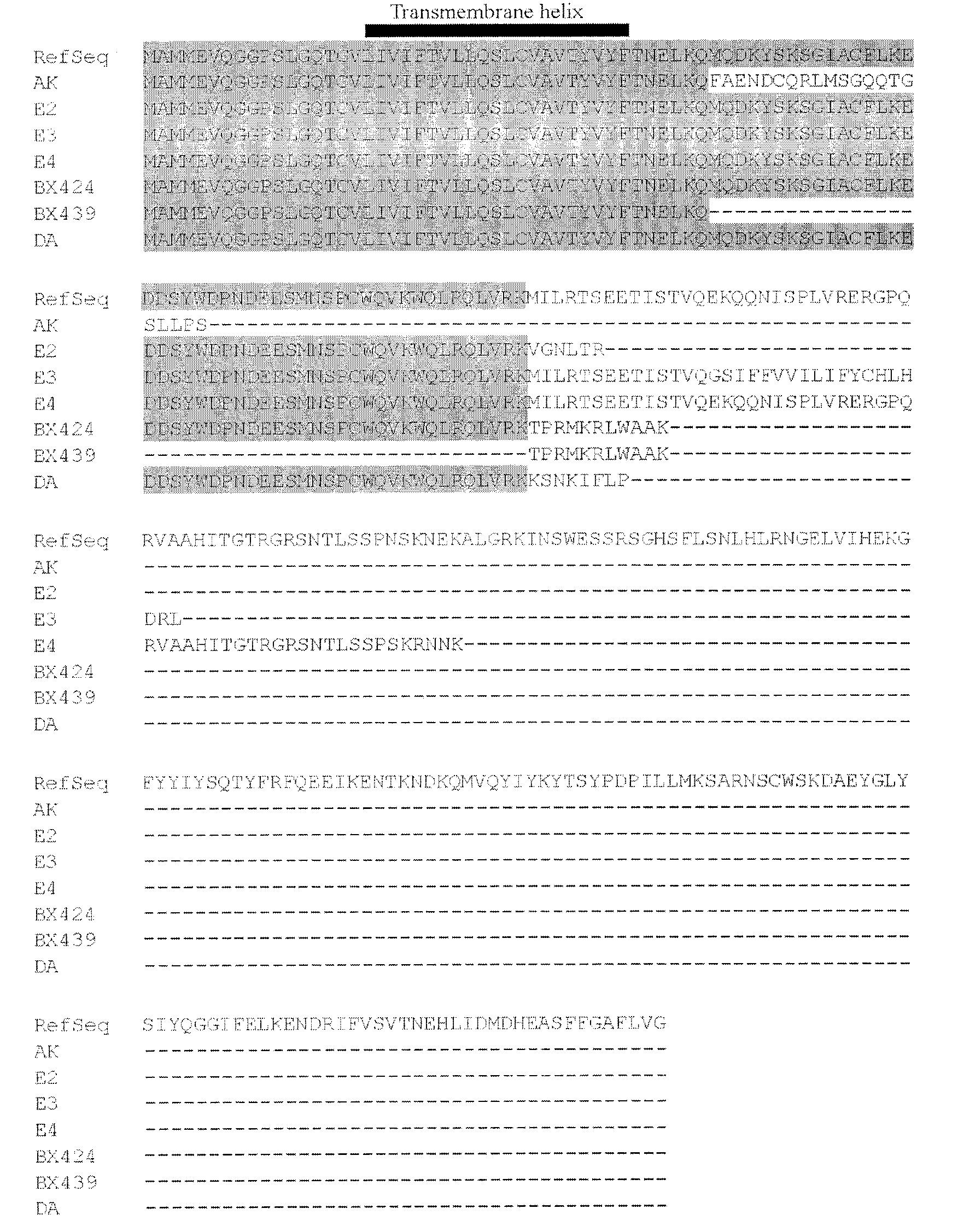
The invention belongs to the field of tumor necrosis factor-(TNF-)-related apoptosis-inducing ligand (TRAIL). Especially, the invention relates to 7 truncated mutants (AK, E2, E3, E4, DA, BX424 and BX439) of TRAIL. A protein sequence coded by DA is completely same with that of TRAILbeta, and a protein sequence coded by BX424 is completely same with that of TRAILdelta. As a result of experiments, among the truncated mutants, except BX439, the mutants have different degrees of capabilities for activating NF-kappaB. As a result of further studies, over-expressions of DA, BX424 and E4 assist in substantially activating macrophage inflammatory protein-1beta(MIP-1beta / CCL4), macrophage inflammatory protein-3alpha(MIP-3alpha / CCL20), and interleukin8(IL8 / CXCL8) promoter reporter gene. The TRAIL mutant provided by the invention has wide application prospect in treating inflammatory responses.
Owner:SINOGENOMAX
Method for promoting proliferation of intestinal cancer cells based on CCL20 and CXCL8
PendingCN112877289AEasy to calculate percent survivalDetermine expression positionGastrointestinal cellsMicrobiological testing/measurementIntestinal CancerStaining
The invention discloses a method for promoting proliferation of intestinal cancer cells based on CCL20 and CXCL8. The method comprises the following steps: 1, selecting a proper experimental material; 2, preparing a main reagent and storing the prepared reagent; 3, proliferating the cells, and carrying out different experimental tests on the proliferated cells; 4, carrying out trypan blue discharge dyeing experiment on the cells, and calculating the survival rate of the cells; 5, carrying out immunohistochemical staining on the cells. Compared with the prior art, multiple groups of experiments are adopted for comparison, the CCL20 stock solution and the CXCL8 stock solution with different concentrations can be respectively arranged for proliferation culture of the intestinal cancer cells; on one hand, the influence of the chemotactic factor concentration on the proliferation rate of the intestinal cancer cells under the condition of the same chemotactic factor is determined; on the other hand, the influence of a single chemotactic factor and two chemotactic factors on the proliferation rate of the intestinal cancer cells under the same chemotactic factor concentration condition can be determined, and the influence of the chemotactic factor concentration and the chemotactic factor number on the proliferation rate of the intestinal cancer cells can be known.
Owner:云南省肿瘤医院
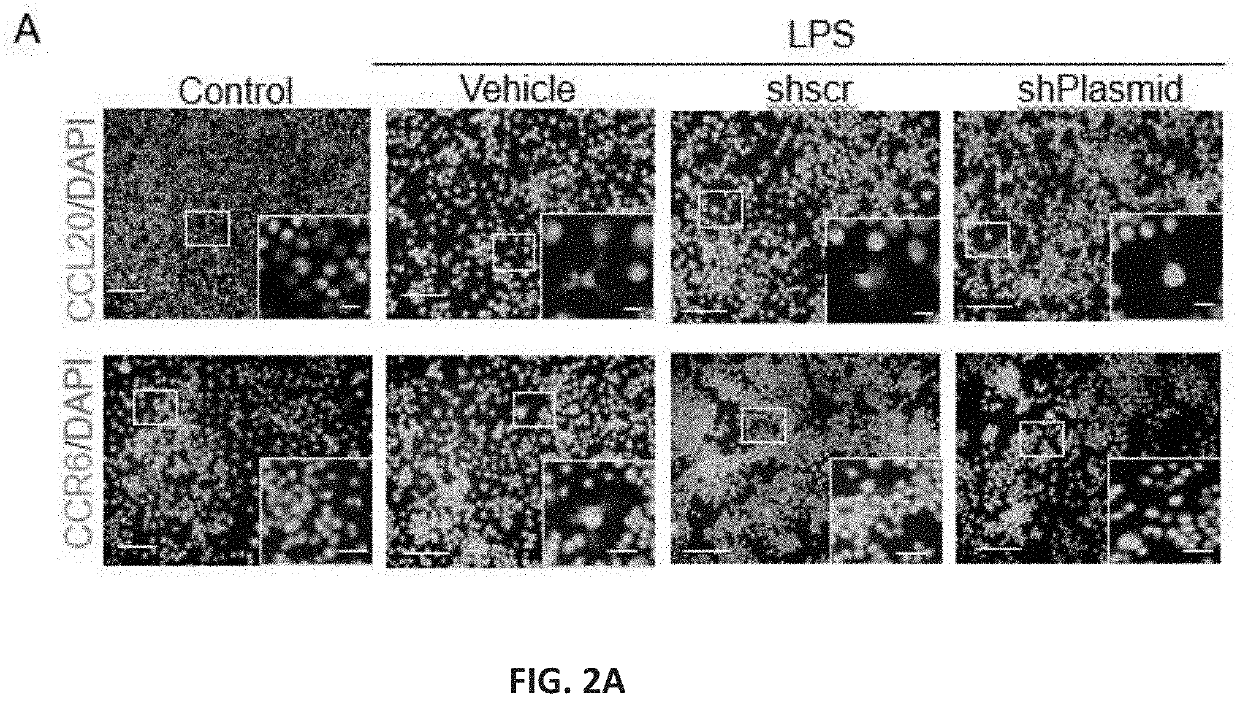
Methods of downregulating ccl20 genes for treatment of traumatic brain injuries
PendingUS20220062382A1Increase heightReduce anxietyOrganic active ingredientsPowder deliveryMicrogliosisDendrite
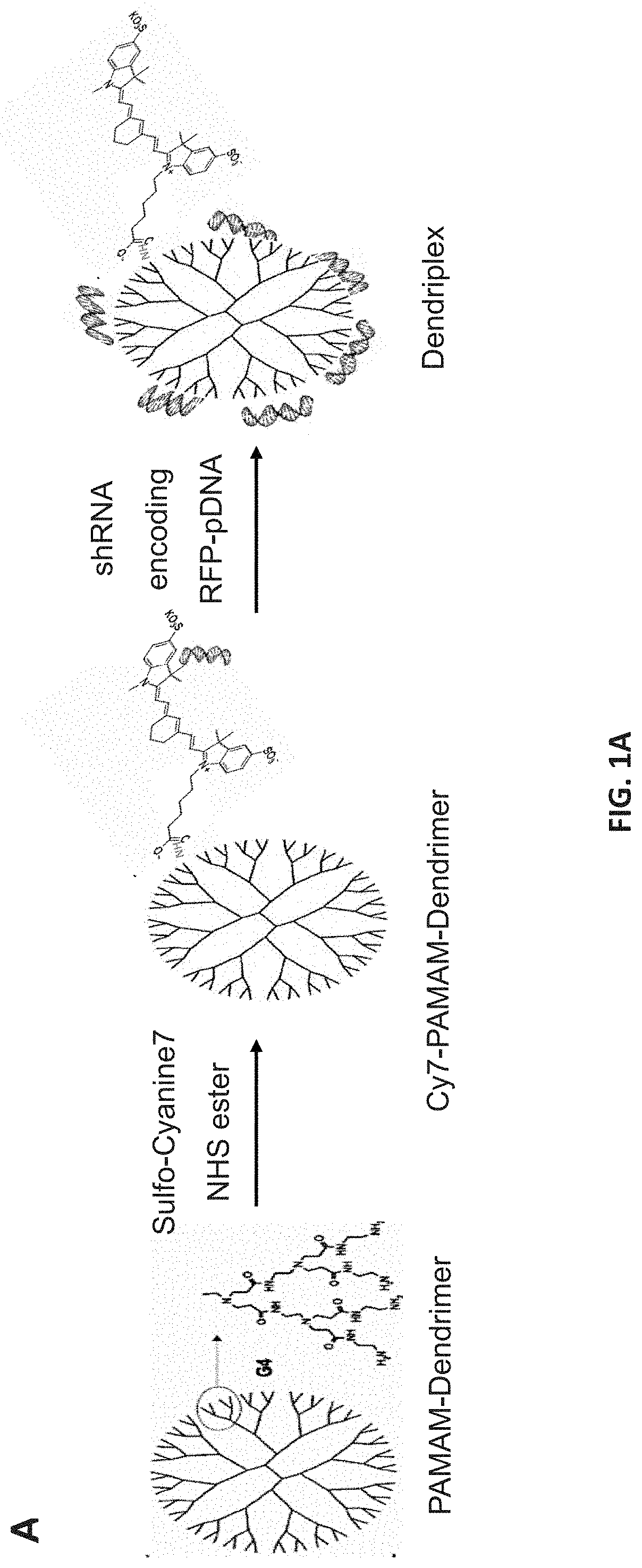
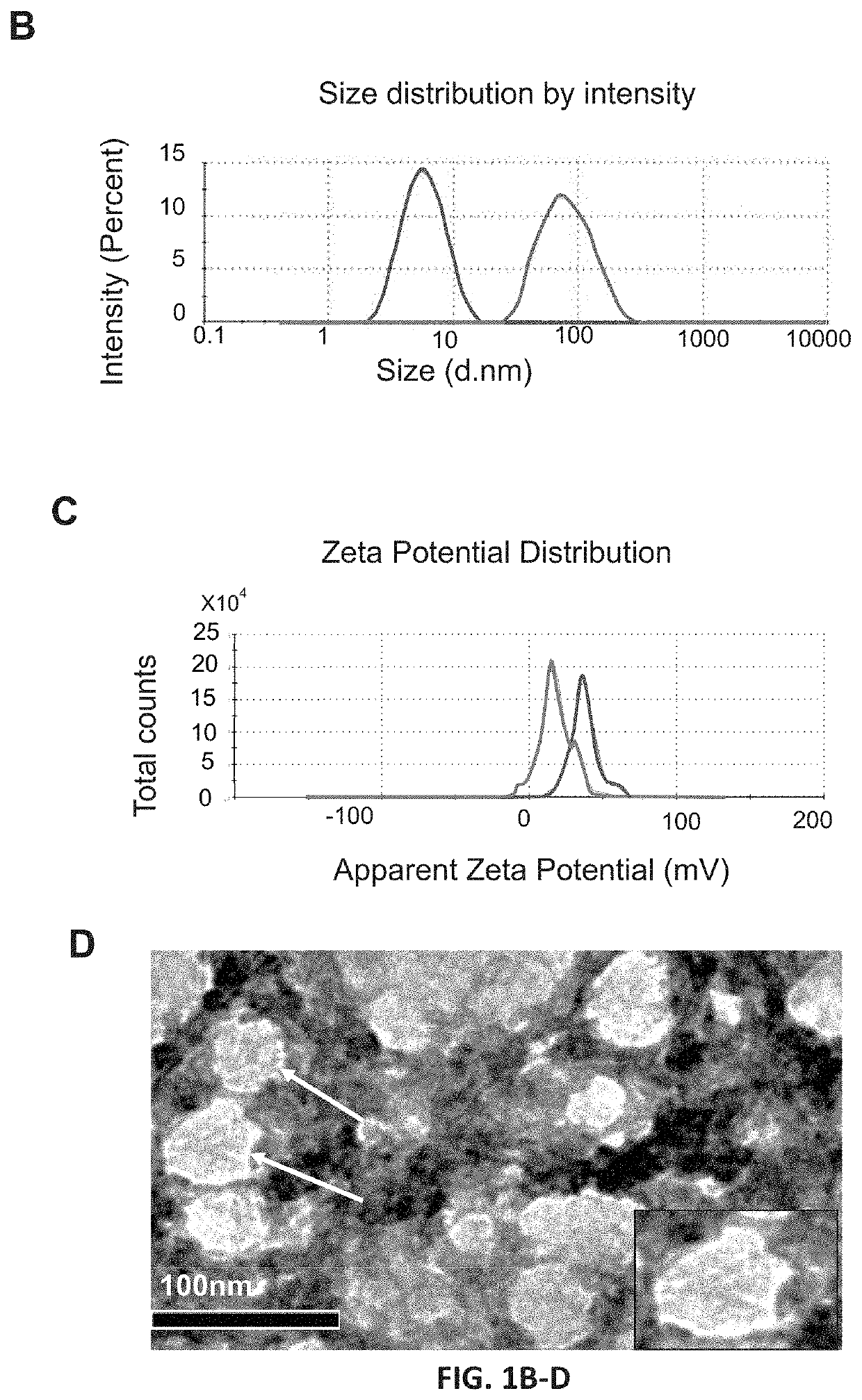
Compositions and methods for treating traumatic brain injury (TBI) are presented. Novel dendriplexes are formed from poly(amidoamine) (PAMAM) dendrimers complexed with shRNA encoding DNA plasmids encapsulating shRNA encoding chemokine ligand 20 (CCL20) gene, chemokine receptor 6 (CCR6) gene, or a combination thereof. The dendriplexes are dually administered, both intranasally and intravenously, prior to administration of stem cells, such as human mesenchymal stem cells (hMSCs) for the treatment of traumatic brain injury (TBI). Administration of the dendriplexes prior to stem cell administration resulted in a decrease in neurodegeneration, neuroinflammation, microgliosis and astrogliosis. In addition, a synergistic increase in brain derived trophic factor (BDNF) was shown by administration of the combination of dendriplex and stem cell administration.
Owner:UNIV OF SOUTH FLORIDA
Immunoadjuvant and its application in diffuse large b-cell lymphoma
ActiveCN110812476BImprove anti-tumor effectReduced proliferation rateCancer antigen ingredientsAntineoplastic agentsOncologyIn vivo
The invention discloses an immune adjuvant for diffuse large B-cell lymphoma based on chemokine CCL20 and its application. It is used in large B-cell lymphoma. The immune adjuvant has a good anti-tumor effect in DLBCL in vitro and in vivo studies. Co-incubation with DLBCL cells can significantly reduce the cell proliferation rate of DLBCL. It acts as an immune adjuvant and inactivates tumors. After the mixed local injection of cells, the mice were generally in good condition, with no abnormal conditions such as convulsions, agitation, piloerection, and convulsions. There were no abnormalities such as redness, inflammation, ulceration, and induration at the local injection site, and non-tumor-bearing mice did not die. , suggesting that it can be used as an immune adjuvant for DLBCL, which can be used in clinical practice and has broad prospects.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Endometrioid adenocarcinoma prognosis-related genes and proteins and their application
Owner:PEOPLES HOSPITAL PEKING UNIV
Prognostic difference genes and recurrence prediction model in early gastric cancer
ActiveCN114107515BIncreased sensitivityAdjust the frequency of follow-up visitsMicrobiological testing/measurementDNA/RNA fragmentationRecurrence predictionGastric carcinoma
This application involves the establishment of a recurrence prediction model for early gastric cancer. By using two batches of gene chip transcriptome data, GSE130823 and GSE55696, a total of 25 genes potentially related to early gastric cancer recurrence were screened out, and AREG, LOC100507520, MMD, CH3L1, A recurrence prediction model based on eight genes: FOS, CCL20, CXCR2, and BATF3. This model has excellent sensitivity, that is, all patients predicted to have no recurrence have no recurrence, and the clinical significance is that the follow-up frequency of these patients can be adjusted accordingly.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
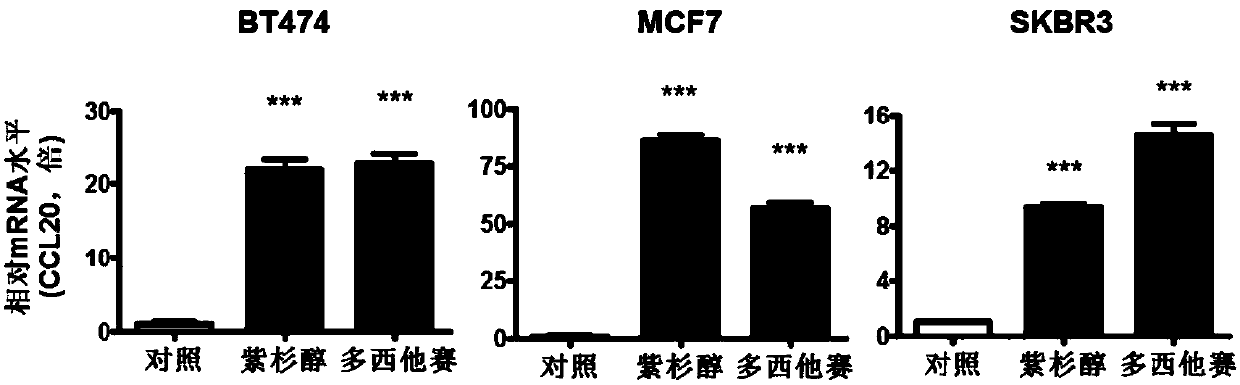
Application of CCL20 to evaluation of therapeutic effect of tumor chemotherapy and tumor therapy
ActiveCN107604064AAccurate Prognostic DiagnosisMicrobiological testing/measurementAntineoplastic agentsMedicineTumor therapy
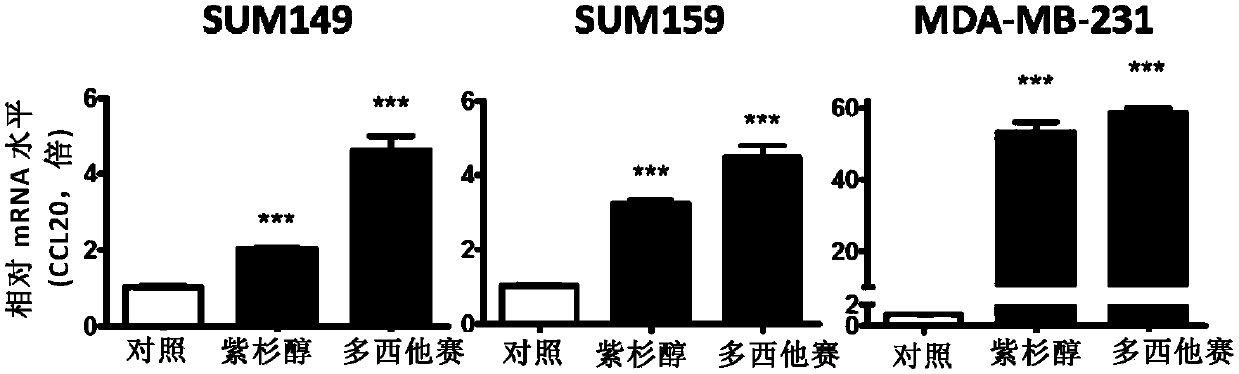
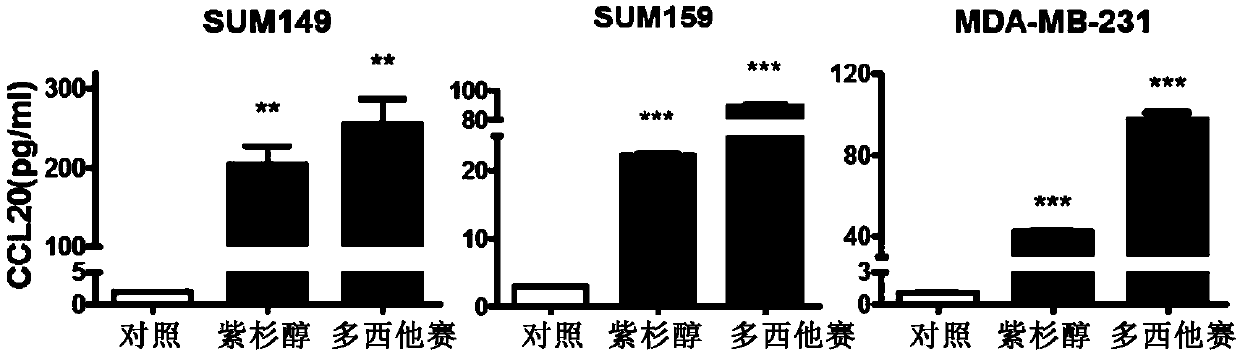
The invention relates to application of CCL20 to the evaluation of the therapeutic effect of tumor chemotherapy and the tumor therapy. Particularly, the invention provides application of a CCL20 gene,mRNA, cDNA, a protein or a detection reagent thereof to preparing a diagnostic reagent or a kit for detecting the drug resistance of a taxane drug. The researches show that the CCL20 can serve as a marker to detect the drug resistance of the taxane drug, and / or can also serve as a target to weaken the drug resistance of the taxane drug. The invention further provides a CCL20-based method for weakening the drug resistance of the taxane drug and a drug composition.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com