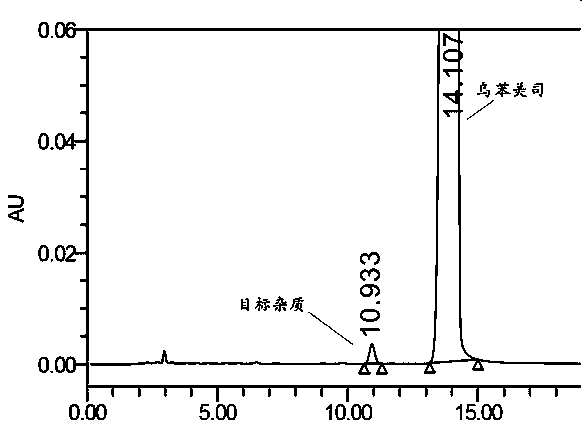
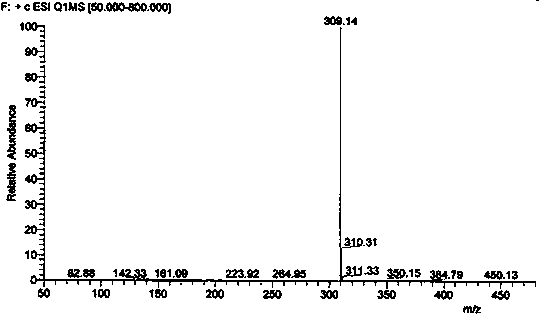
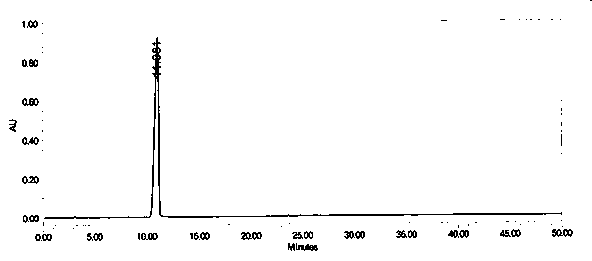
Impurity in ubenimex synthesis and production method thereof
A technology of Ubenimex and impurities, which is applied in the field of drug synthesis, and can solve problems such as difficult quality control and difficult structure confirmation of Ubenimex raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of Example 1 Ubenimex, Collection of Compounds of Formula II
[0029] i Preparation of Ubenimex Crude Product
[0030] Add 1000 mL of tetrahydrofuran into the three-necked flask, start stirring, add 100 g of Z-AHPA, 131.5 g of L-leucine benzyl ester p-toluenesulfonate and 48.5 g of 1-hydroxybenzotriazole (HOBt) in sequence, and then cool down to 0 ±2°C. Add 33.6g of triethylamine and 74.5g of dicyclohexylcarbodiimide (DCC), control the temperature at 12±2°C after the addition, stir and react for 20-24 hours, and filter the reaction solution after the reaction is complete. The filtrate was concentrated under reduced pressure until no distillate was evaporated to obtain an oily substance, and then ethyl acetate was added and stirred until the oily substance was completely dissolved. Wash the above ethyl acetate solution with 650 mL of 0.5 mol / L hydrochloric acid, 650 mL of purified water, 650 mL of saturated sodium bicarbonate solution, and 650 mL of saturated...
Embodiment 2
[0036] The synthesis of embodiment 2 formula II compound
[0037] Preparation of i intermediate formula V compound
[0038] Add 226mL of tetrahydrofuran into a three-neck flask, add 20g of Z-AHPA, 26.3g of L-isoleucine benzyl ester p-toluenesulfonate and 9.7g of 1-hydroxybenzotriazole (HOBt) successively while stirring, and then cool down to 0± 2°C, add 6.72g of triethylamine and 14.9g of dicyclohexylcarbodiimide (DCC), after the addition, control the temperature at 12±2°C, stir and react for 20-24 hours, monitor by HPLC until the reaction is complete, filter the reaction solution , and the filter cake was washed with 25 mL of tetrahydrofuran. The filtrate and washing liquid were combined and then concentrated under reduced pressure until no fraction was distilled off to obtain an oily substance. Add 260 mL of ethyl acetate to dissolve the oily substance, and successively add 133.3 mL of 0.5 mol / L hydrochloric acid, 133.3 mL of purified water, and 133.3 mL of saturated sodium...
Embodiment 3
[0043] The synthesis of embodiment 3 formula II compound
[0044] Preparation of i intermediate formula V compound
[0045] Add 226mL of tetrahydrofuran into the three-necked flask, add Z-AHPA20g, L-isoleucine benzyl p-toluenesulfonate 26.3g and 1-hydroxybenzotriazole (HOBt)9.7g, PyBOP37.3g successively while stirring, and then Cool down to 0±2°C, add DIPEA8.58g, control the temperature at 12±2°C after the addition, stir and react for 20-24 hours, monitor by HPLC until the reaction is complete, filter the reaction solution, and wash the filter cake with 25mL of tetrahydrofuran. The filtrate and washing liquid were combined and then concentrated under reduced pressure until no fraction was distilled off to obtain an oily substance. Add 260 mL of ethyl acetate to dissolve the oily substance, and successively add 133.3 mL of 0.5 mol / L hydrochloric acid, 133.3 mL of purified water, and 133.3 mL of saturated sodium bicarbonate solution. mL, 133.3 mL of saturated sodium chloride so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com