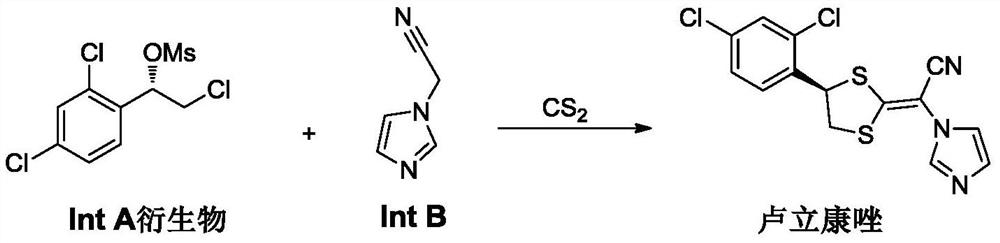
A kind of preparation method of imidazolyl acetonitrile compound
A technology of acetonitrile and imidazole, which is applied in the field of preparation of imidazolyl acetonitrile compounds, can solve the problems of unstable yield and unsuitability for industrial production, and achieve the effects of improving product quality, avoiding the use of high-risk reagents, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
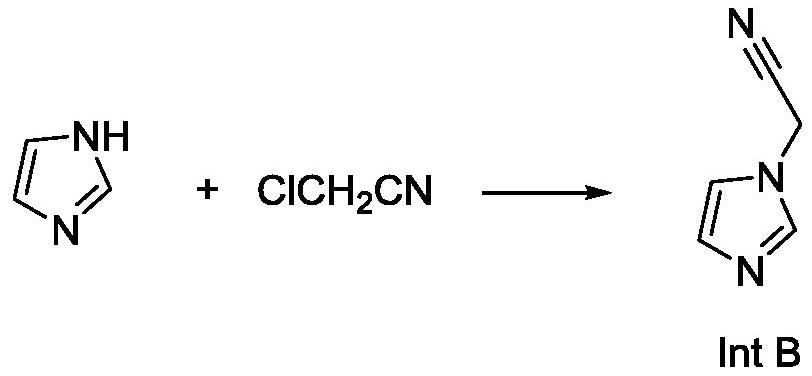
[0050] Example 1 Preparation of 2-(1H-imidazol-1-yl)acetonitrile
[0051] In a 10L three-necked flask, add 5L of acetonitrile and imidazole (1kg, 14.7mol), stir to dissolve them completely, and then cool to 0-10°C. Solid potassium carbonate (1.0 kg, 7.25 mol) and solid KOH (0.5 kg, 8.9 mol) were added. Chloroacetonitrile (1.2kg, 15.9mol) was then added dropwise to the reaction system at a rate to keep the system temperature between 0-5°C. After the addition, the reaction of raw materials is completed. Filter to remove the filter residue, pass through the filtrate with hydrogen chloride gas to precipitate a large amount of solids, and filter to obtain the hydrochloride salt of 2-(1H-imidazol-1-yl)acetonitrile. Then use 5L DCM to beat the wet product, then feed ammonia to make the pH of the system reach 9-10. Filter to remove insoluble matter, evaporate the organic phase to dryness under reduced pressure, add 5 L of methyl tert-butyl ether, cool to below -20 degrees, a large ...
Embodiment 2
[0052] Example 2 Preparation of 2-(1H-imidazol-1-yl)acetonitrile
[0053] In a 10L three-necked flask, add 5L of acetonitrile and imidazole (1kg, 14.7mol), stir to dissolve them completely, and then cool to 0-10°C. Solid potassium carbonate (1.2 kg, 8.7 mol) and potassium hydroxide (0.6 kg, 10.7 mol) were added. Chloroacetonitrile (1.2kg, 15.9mol) was then added dropwise to the reaction system at a rate to keep the system temperature between 0-5°C. After the addition, continue the reaction until the raw material disappears completely. Filter to remove the filter residue, pass through the filtrate with hydrogen chloride gas to precipitate a large amount of solids, and filter to obtain the hydrochloride salt of 2-(1H-imidazol-1-yl)acetonitrile. Then beat the wet product with 5LDCM, then feed ammonia to make the pH of the system reach 9-10. Filter to remove insoluble matter, evaporate the organic phase to dryness under reduced pressure, add 5 L of methyl tert-butyl ether, cool...
Embodiment 3
[0054] Example 3 Preparation of 2-(1H-imidazol-1-yl)acetonitrile
[0055] In a 10L three-necked flask, add 5L of acetonitrile and imidazole (1kg, 14.7mol), stir to dissolve them completely, and then cool to 0-10°C. Solid potassium carbonate (1 kg, 7.3 mol) and solid KOH (1 kg, 17.8 mol) were added. Chloroacetonitrile (1.2kg, 15.9mol) was then added dropwise to the reaction system at a rate to keep the system temperature between 0-5°C. After the addition, the reaction of raw materials is completed. Filter to remove the filter residue, pass through the filtrate with hydrogen chloride gas to precipitate a large amount of solids, and filter to obtain the hydrochloride salt of 2-(1H-imidazol-1-yl)acetonitrile. Then use 5L DCM to beat the wet product, then feed ammonia to make the pH of the system reach 9-10. Filter to remove insoluble matter, evaporate the organic phase to dryness under reduced pressure, add 5 L of methyl tert-butyl ether, cool to below -20 degrees, a large amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com