Synthesis method of 3-chloromethylpyridine hydrochloride
The technology of a chloromethylpyridine hydrochloride and a synthesis method is applied in the field of synthesis of 3-chloromethylpyridine hydrochloride, can solve the problems of expensive reagents, high reagent toxicity, etc., and achieves low cost and few reaction steps. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
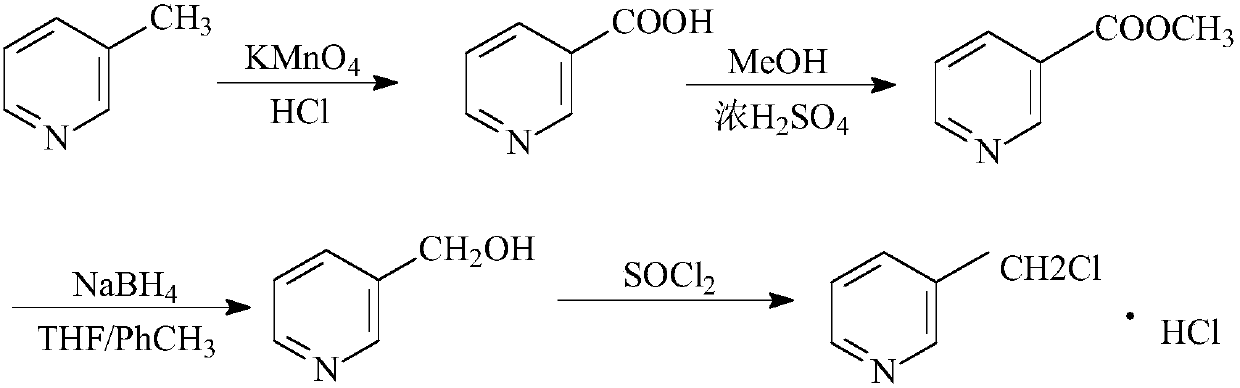
[0021] (1) Add 3-picoline (18.6g, 0.2mol) and 150ml of water into a 250ml flask, heat to 80°C, add potassium permanganate (66.36g, 0.42mol) in batches, and maintain the temperature at 85- Heat and stir at 90°C for 30min, track and analyze by thin layer chromatography, adjust the pH value of the reaction solution to 3 with 2mol / l hydrochloric acid after the reaction is complete, cool the temperature of the reaction solution to 25°C and suction filter to obtain 3-pyridinecarboxylic acid.
[0022] (2) 3-pyridinecarboxylic acid and methanol (12g, 0.26mol) were reacted with concentrated sulfuric acid to generate 3-picolinic acid methyl ester.
[0023] (3) Methyl 3-pyridinecarboxylate was dissolved in a solvent mixed with 75ml THF and 75ml toluene, and sodium borohydride (30.24g, 0.8mol) and aluminum trichloride were added in batches at 0-5°C, and the reaction continued for 3 -4h, TLC tracking analysis until the reaction is complete to get 3-pyridinemethanol.
[0024] (4) 2-pyridin...
Embodiment 2
[0026] (1) Add 3-picoline (18.6g, 0.2mol) and 150ml of water into a 250ml flask, heat to 80°C, add potassium permanganate (69.5g, 0.44mol) in batches, and maintain the temperature at 85- Heat and stir at 90°C for 30 minutes, follow-up analysis by thin-layer chromatography, adjust the pH value of the reaction solution to 4 with 2mol / l hydrochloric acid after the reaction is complete, cool the temperature of the reaction solution to 25°C and suction filter to obtain 3-pyridinecarboxylic acid.
[0027] (2) 3-pyridinecarboxylic acid and methanol (12g, 0.26mol) were reacted with concentrated sulfuric acid to generate 3-picolinic acid methyl ester.
[0028] (3) Methyl 3-pyridinecarboxylate was dissolved in a solvent mixed with 75ml THF and 75ml toluene, and sodium borohydride (34g, 0.9mol) and aluminum trichloride were added in batches at 0-5°C, and the reaction was continued after adding 3- 4h, TLC follow-up analysis until the reaction is complete to get 3-pyridinemethanol.
[002...
Embodiment 3
[0031] (1) Add 3-picoline (18.6g, 0.2mol) and 150ml of water into a 250ml flask, heat to 80°C, add potassium permanganate (72.7g, 0.46mol) in batches, and maintain the temperature at 85- Heating and stirring at 90°C for 30 minutes, followed by thin-layer chromatography analysis, after the reaction is complete, use 2mol / l hydrochloric acid to adjust the pH value of the reaction solution to 4, and cool the temperature of the reaction solution to 25°C to obtain 3-pyridinecarboxylic acid by suction filtration.
[0032] (2) 3-pyridinecarboxylic acid and methanol (12g, 0.26mol) were reacted with concentrated sulfuric acid to generate 3-picolinic acid methyl ester.
[0033] (3) Methyl 3-pyridinecarboxylate was dissolved in a solvent mixed with 75ml THF and 75ml toluene, and sodium borohydride (37.8g, 1.0mol) and aluminum trichloride were added in batches at 0-5°C, and the reaction continued for 3 -4h, TLC tracking analysis until the reaction is complete to get 3-pyridinemethanol.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com