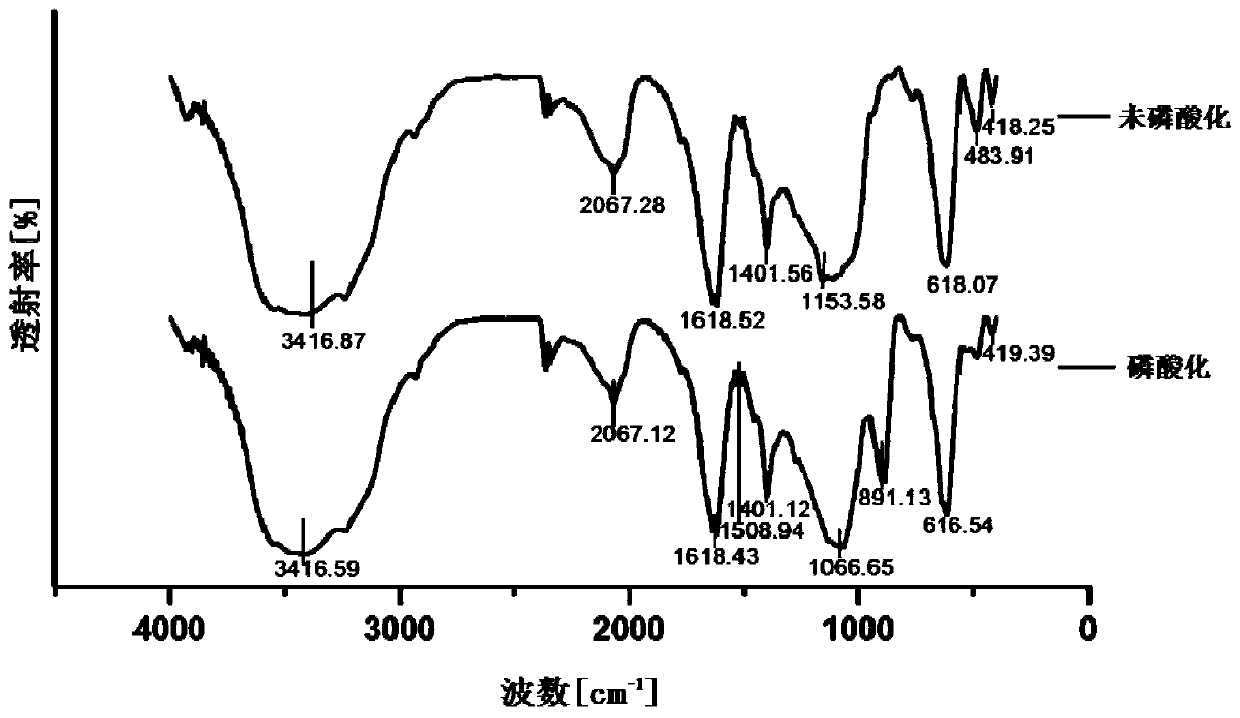
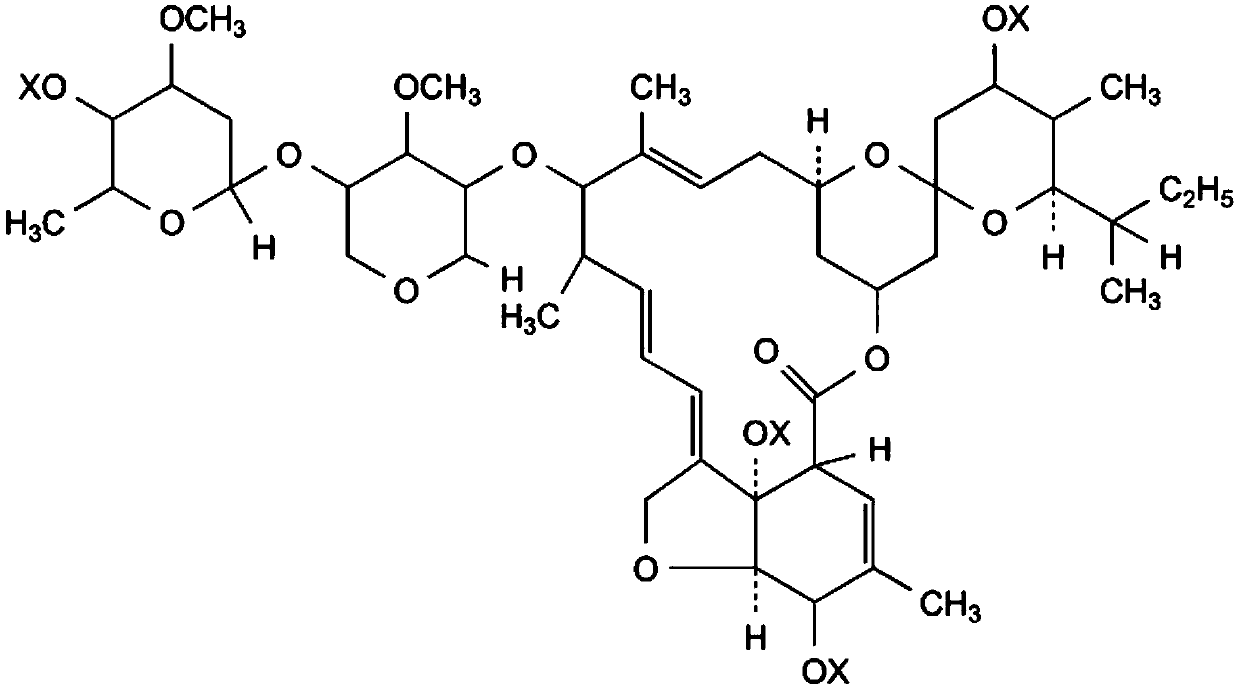
Avermectin B2a phosphorylation modifier as well as preparation method and application thereof
A technology of abamectin and phosphorylation, applied in botany equipment and methods, phosphorus organic compounds, applications, etc., can solve problems such as poor application effect of abamectin B2a
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This example provides a method for preparing a phosphorylated modified abamectin B2a.
[0028] Specifically include the following steps:
[0029] 10g Abamectin B2a is dissolved in the ethanol (95%v / v) of 100ml, the sodium phosphate of 3.5g is added in the solution, stirring and dissolving, adjust pH value to 8 with 5% sodium hydroxide solution, set solution temperature at 60°C, put the solution into the irradiation platform, and irradiate it with an electron beam (electron beam 10MeV), with an irradiation dose of 1.50kGy. After the irradiation, control the reaction time for 2h, and then evaporate the ethanol on a rotary evaporator , to precipitate the precipitate, filter the precipitate, and dry in a vacuum drying oven at a temperature not exceeding 60°C. Add 30ml of ethanol to the dried solid for recrystallization to obtain the phosphorylated avermectin B2a.
Embodiment 2
[0031] This example provides a method for preparing a phosphorylated modified abamectin B2a.
[0032] Specifically include the following steps:
[0033] 10g Abamectin B2a is dissolved in the ethanol (92%v / v) of 100ml, adds the sodium phosphate of 7g in the solution, stirs and dissolves, adjusts pH value to 6 with 5% sodium hydroxide solution, setting solution temperature is 40°C, put the solution into the irradiation platform, and irradiate it with an electron beam (electron beam 10MeV), with an irradiation dose of 1.00kGy. After the irradiation, control the reaction time for 0.5h, and then evaporate the ethanol on a rotary evaporator , to precipitate the precipitate, filter the precipitate, and dry in a vacuum drying oven at a temperature not exceeding 60°C. Add 30ml of ethanol to the dried solid for recrystallization to obtain the phosphorylated avermectin B2a.
Embodiment 3
[0035] This example provides a method for preparing a phosphorylated modified abamectin B2a.
[0036] Specifically include the following steps:
[0037] 10g Abamectin B2a is dissolved in the ethanol (95%v / v) of 100ml, in the solution, add the sodium phosphate of 3g, stirring and dissolving, adjust pH value to 8 with 5% sodium hydroxide solution, set solution temperature as 60°C, put the solution into the irradiation platform, and irradiate it with an electron beam (electron beam 10MeV), with an irradiation dose of 1.50kGy. After the irradiation, control the reaction time for 1.5h, and then evaporate the ethanol on a rotary evaporator , to precipitate the precipitate, filter the precipitate, and dry in a vacuum drying oven at a temperature not exceeding 60°C. Add 30ml of ethanol to the dried solid for recrystallization to obtain the phosphorylated avermectin B2a.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com