In vitro dual-dynamic degradation performance monitoring device for medical magnesium alloy implantable device
A technology for magnesium alloys and devices, which is applied in the field of devices for monitoring the dual dynamic degradation performance of medical magnesium alloy implants in vitro. The effect of contact error, reducing error interference, and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] The technical solution of the present invention will be further described below in conjunction with the accompanying drawings.
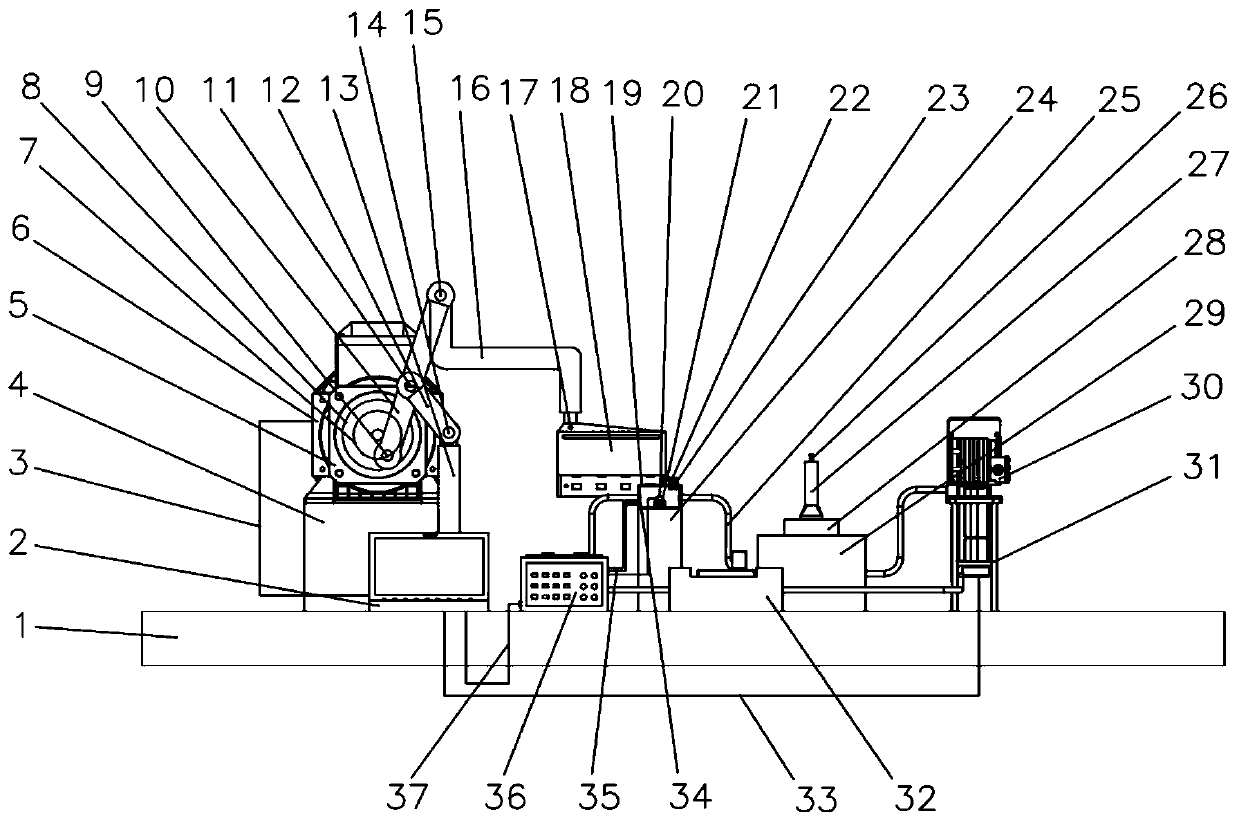
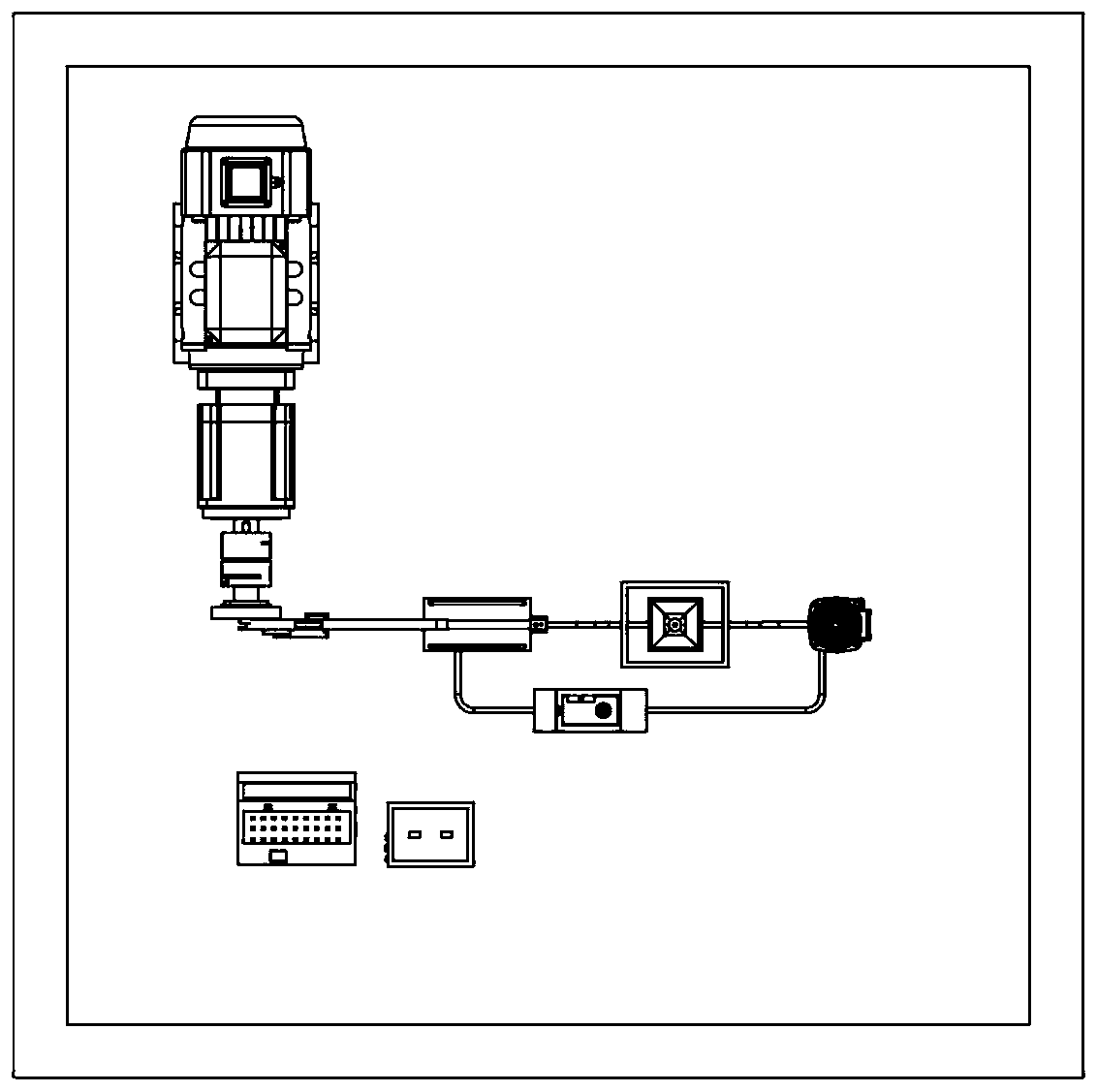
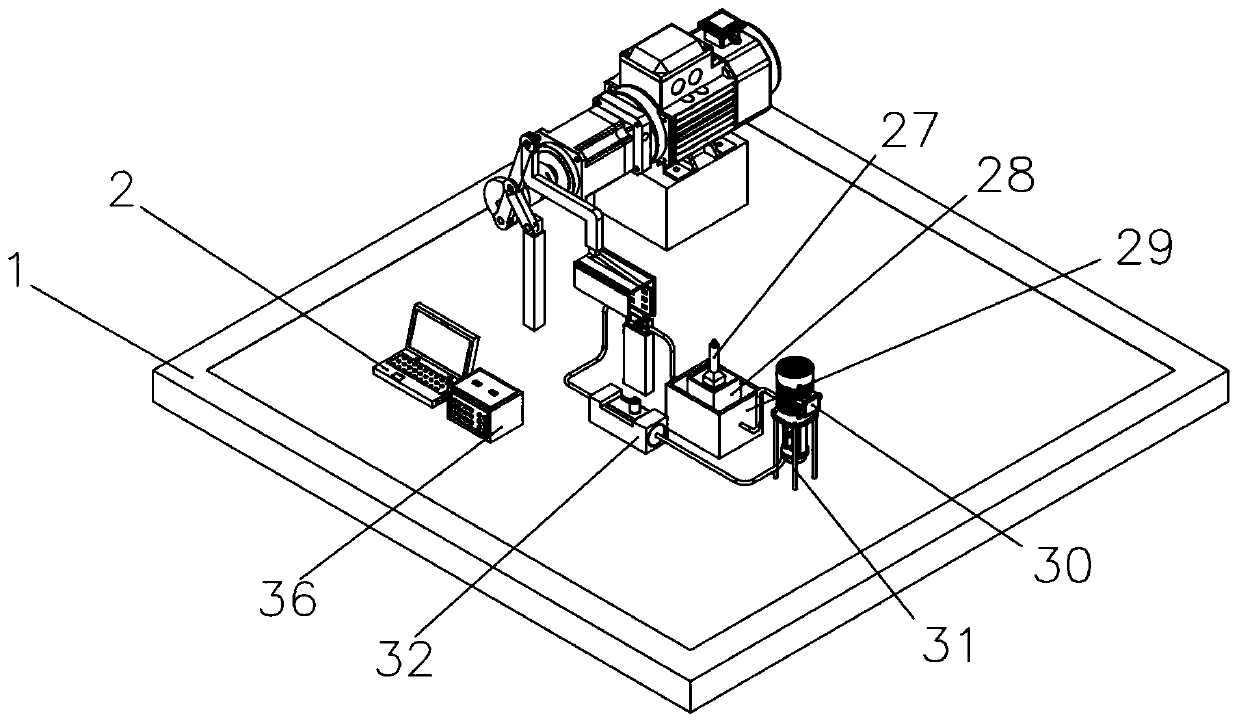
[0058] see figure 1 , image 3 , Figure 4 , Figure 5 The device of the present invention consists of a base 1, a computer 2, a first control line 3, a motor support base 4, a reducer 5, a motor 6, a shaft coupling 7, a rotating disk 8, a first pin 9, a first connecting rod 10, Second pin 11, second connecting rod 12, second connecting rod support frame 13, third pin 14, fourth pin 15, third connecting rod 16, bolt 17, active magnetic assembly 18, sample slot 19, to be tested Device 20, driven magnetic assembly 21, pH meter 22, thermometer 23, sample tank support seat 24, pipe 25, hydrogen sensor 26, measuring cylinder 27, liquid storage tank 28, constant temperature tank 29, circulation pump 30, circulation pump support frame 31. A flow meter 32, a second control line 33, an electrode 34, a test line 35, an electrochemical workstation 36...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com