Green synthesis method of 1, 4-diamino-anthraquinone
A green synthesis technology of diaminoanthraquinone, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as high price, high toxicity of hydrazine hydrate, difficult treatment of waste acid and high-salt wastewater, etc. Achieve the effect of low production cost and high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
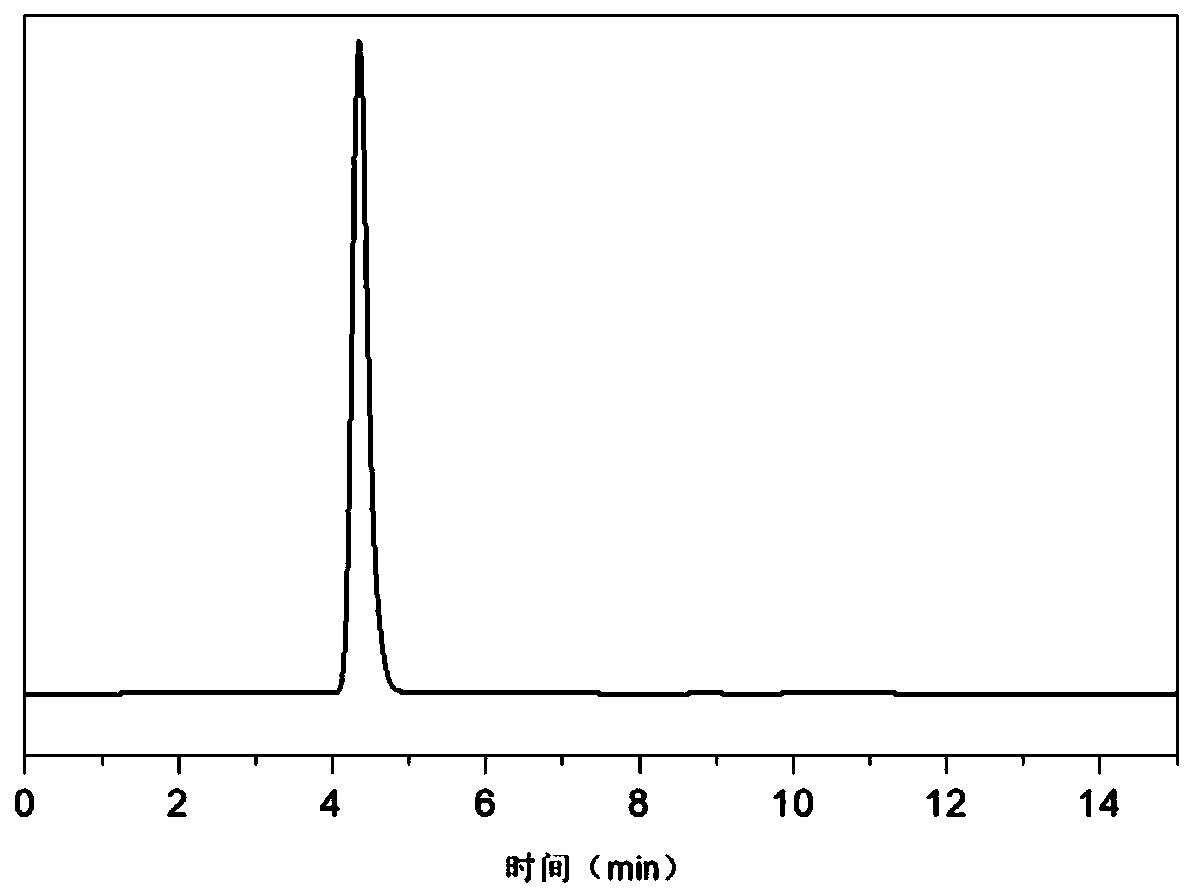
[0021] Add 8g of 1,4-dihydroxyanthraquinone, 100mL of toluene and 0.6g of Pd with a palladium content of 1% into a 500mL autoclave 10 Ru 1 / C catalyst, the catalytic hydrogenation reaction is carried out under the conditions of the reaction temperature of 40°C and the reaction pressure of 1.0MPa. After the reaction is completed, the catalyst is filtered to obtain the hydrogenation liquid. The hydrogenation solution reacts with ammonia for 2 hours. After the reaction, excess ammonia is discharged. After heating and evaporating toluene, the 1,4-diaminoanthraquinone leuco is obtained, and then the 1,4-diaminoanthraquinone leuco is obtained in the air. The 1,4-diaminoanthraquinone was obtained after the color body was heated for 1 hour. HPLC analysis product purity 98.3%, calculated yield is 95%, as figure 1 shown.
Embodiment 2
[0023] 10g of 1,4-dihydroxyanthraquinone, 200mL of ethanol and 1.0g of Pd with a palladium content of 1% were put into a 500mL autoclave 1 Ru 1 / C catalyst, the catalytic hydrogenation reaction is carried out under the conditions of the reaction temperature of 90°C and the reaction pressure of 0.4MPa. After the reaction is completed, the catalyst is filtered to obtain the hydrogenation liquid. The hydrogenation solution reacts with ammonia for 4 hours. After the reaction, excess ammonia is discharged. After heating and evaporating ethanol, the 1,4-diaminoanthraquinone leuco is obtained, and then the 1,4-diaminoanthraquinone leuco is obtained in the air. The 1,4-diaminoanthraquinone was obtained after the color body was heated for 1 hour. The purity of the product analyzed by HPLC was 96.7%, and the calculated yield was 96%.
Embodiment 3
[0025] Put 40g of 1,4-dihydroxyanthraquinone, 300mL of toluene and 2g of PdRu with a palladium content of 1% into a 1000mL autoclave 2 / C catalyst, the catalytic hydrogenation reaction is carried out at a reaction temperature of 60°C and a reaction pressure of 0.6MPa. After the reaction is completed, the catalyst is filtered to obtain a hydrogenation liquid. Under the conditions of a reaction temperature of 60°C and a reaction pressure of 0.6MPa, The hydrogenation solution reacts with ammonia for 1 hour. After the reaction, the ammonia gas is discharged. After heating and evaporating toluene, the 1,4-diaminoanthraquinone leuco is obtained, and then the 1,4-diaminoanthraquinone leuco is obtained in the air. After heating for 3 hours, 1,4-diaminoanthraquinone was obtained. The purity of the product analyzed by HPLC was 97.2%, and the calculated yield was 96%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com