A kind of synthetic method of 1,4-diaminoanthraquinone
A technology of diaminoanthraquinone and synthesis method, which is applied in the field of synthesis of 1,4-diaminoanthraquinone, can solve the problems of high price, high toxicity of hydrazine hydrate, difficult treatment of waste acid and high-salt wastewater, and achieve production cost The effect of low, high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
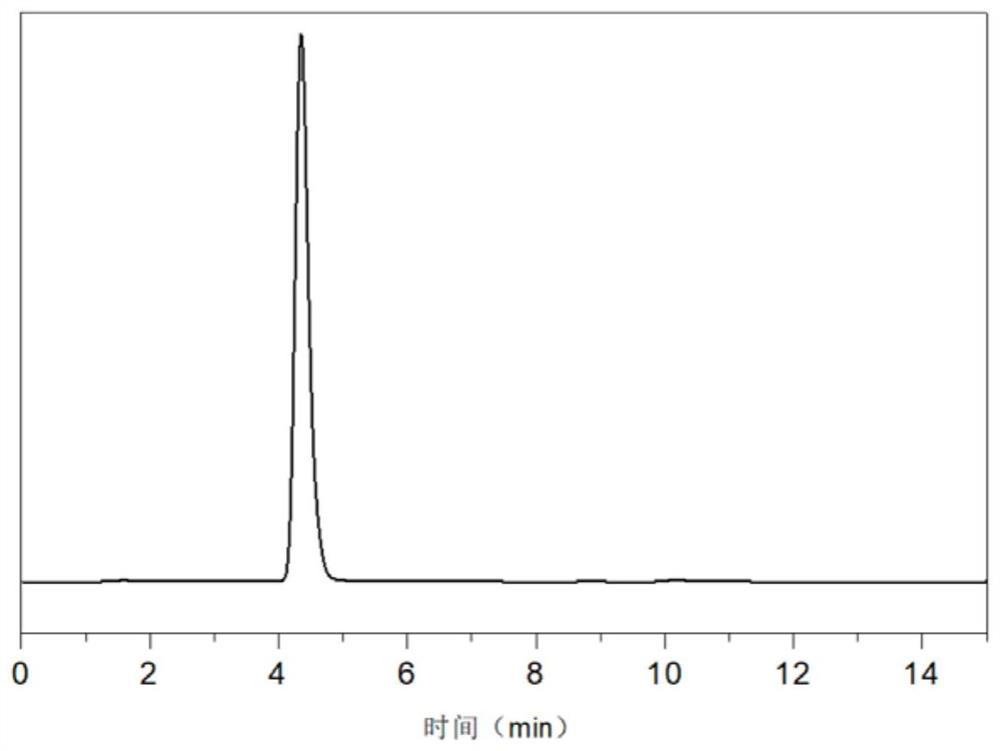
[0021] Add 8g of 1,4-dihydroxyanthraquinone, 100mL of toluene and 0.6g of Pd with a palladium content of 1% into a 500mL autoclave 10 Ru 1 / C catalyst, the catalytic hydrogenation reaction is carried out under the conditions of the reaction temperature of 40°C and the reaction pressure of 1.0MPa. After the reaction is completed, the catalyst is filtered to obtain the hydrogenation liquid. The hydrogenation solution reacts with ammonia for 2 hours. After the reaction, excess ammonia is discharged. After heating and evaporating toluene, the 1,4-diaminoanthraquinone leuco is obtained, and then the 1,4-diaminoanthraquinone leuco is obtained in the air. The 1,4-diaminoanthraquinone was obtained after the color body was heated for 1 hour. HPLC analysis product purity 98.3%, calculated yield is 95%, as figure 1 shown.
Embodiment 2
[0023] 10g of 1,4-dihydroxyanthraquinone, 200mL of ethanol and 1.0g of Pd with a palladium content of 1% were put into a 500mL autoclave 1 Ru 1 / C catalyst, the catalytic hydrogenation reaction is carried out under the conditions of the reaction temperature of 90°C and the reaction pressure of 0.4MPa. After the reaction is completed, the catalyst is filtered to obtain the hydrogenation liquid. The hydrogenation solution reacts with ammonia for 4 hours. After the reaction, excess ammonia is discharged. After heating and evaporating ethanol, the 1,4-diaminoanthraquinone leuco is obtained, and then the 1,4-diaminoanthraquinone leuco is obtained in the air. The 1,4-diaminoanthraquinone was obtained after the color body was heated for 1 hour. The purity of the product analyzed by HPLC was 96.7%, and the calculated yield was 96%.
Embodiment 3
[0025] Put 40g of 1,4-dihydroxyanthraquinone, 300mL of toluene and 2g of PdRu with a palladium content of 1% into a 1000mL autoclave 2 / C catalyst, the catalytic hydrogenation reaction is carried out at a reaction temperature of 60°C and a reaction pressure of 0.6MPa. After the reaction is completed, the catalyst is filtered to obtain a hydrogenation liquid. Under the conditions of a reaction temperature of 60°C and a reaction pressure of 0.6MPa, The hydrogenation solution reacts with ammonia for 1 hour. After the reaction, the ammonia gas is discharged. After heating and evaporating toluene, the 1,4-diaminoanthraquinone leuco is obtained, and then the 1,4-diaminoanthraquinone leuco is obtained in the air. After heating for 3 hours, 1,4-diaminoanthraquinone was obtained. The purity of the product analyzed by HPLC was 97.2%, and the calculated yield was 96%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com