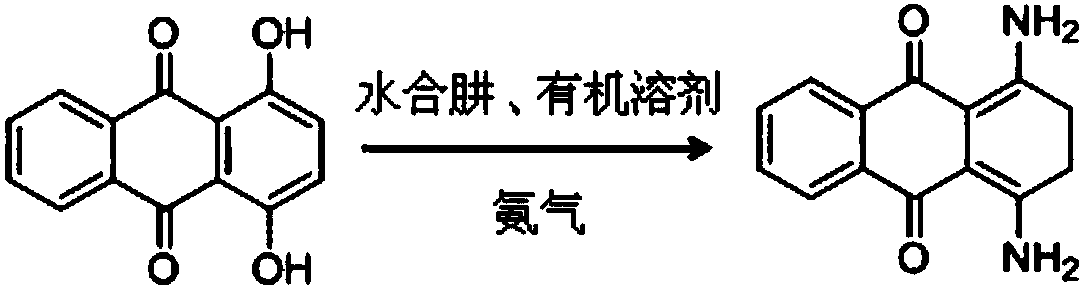
Synthesis method of 1,4-diamino-anthraquinone leuco body
A technology of diaminoanthraquinone and synthesis method, which is applied in the field of synthesis of 1,4-diaminoanthraquinone leuco, can solve the problems of difficult treatment, dark wastewater, large ammonia consumption, etc., to reduce production costs, The effect of increasing product yield and reducing ammonia consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 17.7g of hydrazine hydrate (85%, 0.3mol), 120g of 1,4-dihydroxyanthraquinone (0.5mol), and 400g of ethylene glycol monomethyl ether into a 1000ml autoclave. Close the autoclave, replace with nitrogen once and once with ammonia. Turn on stirring, feed ammonia gas, and adjust the pressure to 0.3Mpa. Heat up to 80°C and keep the pressure at 0.3Mpa. React for about 3 to 4 hours, pass 28 to 32 g of ammonia in total, close the ammonia gas inlet, slowly open the exhaust port, and gradually discharge excess ammonia to absorb water until the pressure is zero. Raise the temperature to 100°C and continue to stir to remove ammonia for 1h, stir and cool, then cool down to 5-10°C for about 1h, and keep it for 1h. After filtering, the filter cake was washed with 50 g of water, and air-dried at 70-75° C. for 5 h to obtain 116 g of 1,4-dihydroxyanthraquinone leuco compound, with a purity of 99.3% by HPLC and a calculated yield of 95.7%.
Embodiment 2
[0024] Add 17.7g of hydrazine hydrate (85%, 0.3mol), 120g of 1,4-dihydroxyanthraquinone (0.5mol), and 400g of sulfolane into a 1000ml autoclave. Close the autoclave, replace with nitrogen once and once with ammonia. Turn on the stirring, feed ammonia gas, and adjust the pressure to 0.4Mpa. Heat up to 80°C and keep the pressure at 0.4Mpa. React for about 3 to 4 hours, pass 28 to 32 g of ammonia in total, close the ammonia gas inlet, slowly open the exhaust port, and gradually discharge excess ammonia to absorb water until the pressure is zero. Raise the temperature to 100°C and continue to stir to remove ammonia for 1h, stir and cool, then cool down to 5-10°C for about 1h, and keep it for 1h. After filtering, the filter cake was washed with 50 g of water, and air-dried at 70-75° C. for 5 h to obtain 113 g of 1,4-dihydroxyanthraquinone leuco compound. The purity by HPLC was 99.2%, and the calculated yield was 93.4%.
Embodiment 3
[0026] Add 23.6g of hydrazine hydrate (85%, 0.4mol), 120g of 1,4-dihydroxyanthraquinone (0.5mol), and 400g of sulfolane into a 1000ml autoclave. Close the autoclave, replace with nitrogen once and once with ammonia. Turn on the stirring, feed ammonia gas, and adjust the pressure to 0.4Mpa. Heat up to 80°C and keep the pressure at 0.4Mpa. React for about 3 to 4 hours, pass 28 to 32 g of ammonia in total, close the ammonia gas inlet, slowly open the exhaust port, and gradually discharge excess ammonia to absorb water until the pressure is zero. Raise the temperature to 100°C and continue to stir to remove ammonia for 1h, stir and cool, then cool down to 5-10°C for about 1h, and keep it for 1h. After filtering, the filter cake was washed with 50 g of water, and air-dried at 70-75° C. for 5 h to obtain 113 g of 1,4-dihydroxyanthraquinone leuco compound. The purity by HPLC was 99.2%, and the calculated yield was 94.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com