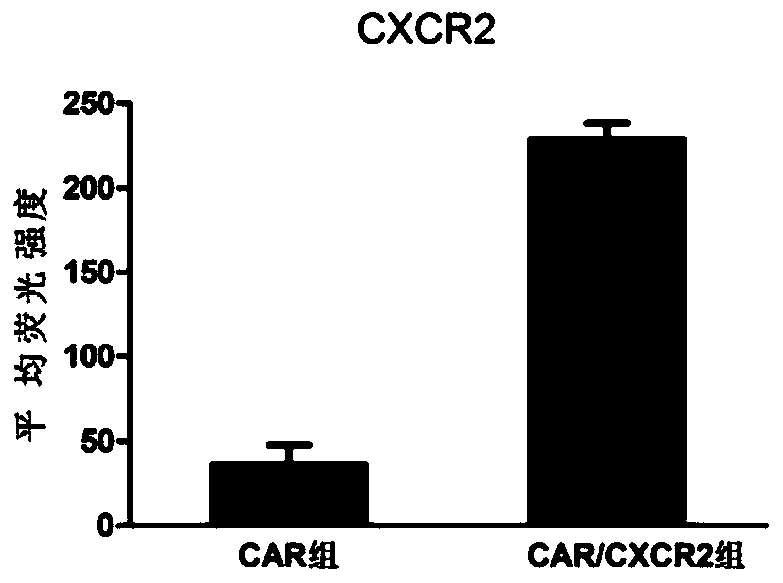
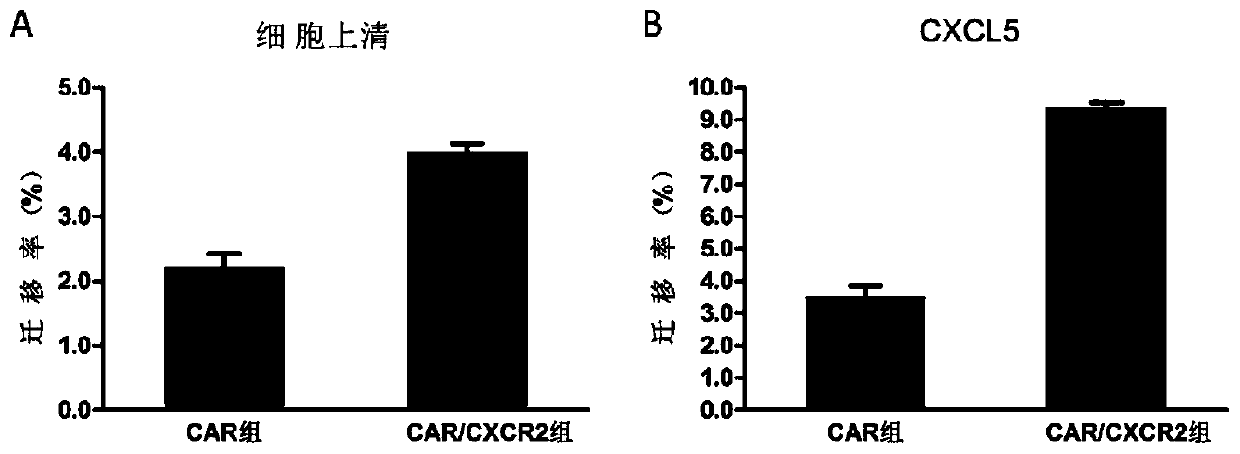
CAR-NK92 cell expressing CXCR2, preparation method and application thereof
A technology of immune cells and preparations, applied in the fields of botanical equipment and methods, biochemical equipment and methods, and cells modified by the introduction of foreign genetic material, etc., can solve the problems of poor treatment effect of solid tumors, cytokine storm, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0193] In a specific embodiment, the present invention provides a method for preparing engineered immune cells, comprising the following steps:
[0194] S1: CD3ζ is used as the intracellular signal segment of CAR, NKG2D is used as the transmembrane region, 4-1BB is used as a co-stimulatory factor, and a variant of the extracellular binding region of human SIRPα is used to recognize CD47, forming a chimeric antigen that specifically recognizes CD47 Receptor, and artificially synthesize the corresponding nucleotide sequence: vSIRPα-CD8-NKG2D-4-1BB-CD3ζ, and connect the CAR and CXCR2 nucleotide sequence through the T2A nucleotide sequence to form a fusion gene: vSIRPα-CD8-NKG2D-4-1BB-CD3ζ-T2A-CXCR2.
[0195] S2: inserting the fusion gene fragment into the lentiviral expression plasmid to obtain the target plasmid. Packaged as a lentivirus carrying vSIRPα-CD8-NKG2D-4-1BB-CD3ζ-T2A-CXCR2
[0196] S3: Take 293FT cells in the logarithmic growth phase and inoculate them into cell cul...
Embodiment 1
[0232] Example 1 Construction of recombinant lentiviral vector pLV-EF1-MCS-vSIRPα-T2A-CXCR2
[0233] Gene synthesis vSIRPα-CD8-NKG2D-4-1BB-CD3ζ-T2A-CXCR2 nucleotide sequence (SEQ ID NO.: 1), its main structure is as follows figure 1 shown. It was connected to the pLV-EF1-MCS plasmid by restriction enzyme transformation, and the upstream of the gene was the EF1a promoter. The vector was transformed into Stb13 Escherichia coli strain, and positive clones were obtained by screening with ampicillin. The plasmid was extracted, the clone was identified by enzyme digestion, and the lentiviral expression plasmid pLV-EF1-MCS-vSIRPα-T2A-CXCR2 was obtained.
Embodiment 2
[0234] The preparation of embodiment 2 lentiviruses
[0235] (1) One day before transfection, 293FT cells in logarithmic growth phase were inoculated in a 10 cm cell culture dish, and transfection was started when the cell density reached about 80%.
[0236] (2) Fully mix sterile water and 2M CaCl2 solution according to the volume of 9:1, the total volume is 500uL, and at the same time add 9ug of lentiviral expression plasmid pLV-EF1-MCS-vSIRPα-T2A-CXCR2, and add 4.63 μg of packaging plasmid PLP1 Add 4.3ug of ug, PLP2, and 3.01ug of VSVG, vortex and mix well, then add them dropwise to 500uL of HEPPS buffer, vortex and mix well, and let stand at room temperature for 20min. Finally, the mixed transfection solution was evenly added to a 10 cm cell culture dish, and placed in a 37° C., 5% CO2 incubator for 12 hours.
[0237] (3) Discard the liquid in the cell culture dish, and replace it with fresh DMEM cell culture medium containing 2% fetal bovine serum to continue culturing. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com