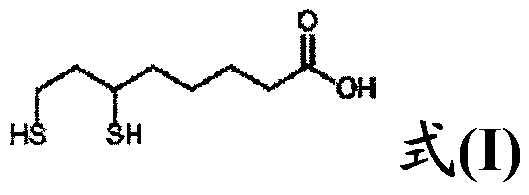
Process for the production of dihydrolipoic acid
A technology of dihydrolipoic acid and dichlorooctanoic acid, which is applied in the direction of mercaptan preparation and organic chemistry, and can solve problems such as low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 123.0 g (500 mmol) of racemic 6,8-dichloroethyl octanoate and 14.4 g (450 mmol, 0.9 equivalent) of sulfur was pre-placed in 123.0 g of ethanol. The autoclave was closed and heated to an internal temperature of 110° C. with stirring. At this temperature, 50.7 g of Na 2 S (650mmol, 1.3 equivalents) of 412.5g aqueous sodium sulfide (12.3% by weight Na in water 2 S). Here, the pressure increased from the initial 0.33MPa to 0.43MPa. Stirring of the reaction mixture was continued at 110 °C for 120 min, then cooled to 50 °C and transferred to a 2000 ml four-necked flask with stirrer, metering pump, internal thermometer, reflux cooler and oil bath heater. A solution of 94.6 g of sodium borohydride (12% by weight) in sodium hydroxide solution (40% by weight) containing 11.4 g of NaBH was uniformly added dropwise within 30 min at 70°C 4 (300 mmol, 0.60 equiv). The ethanol contained was substantially distilled from the reaction mixture over a period of 60 min (up to a distil...
Embodiment 2
[0061] Example 2 (comparative example not according to the invention):
[0062] 123.0 g (500 mmol) of racemic 6,8-dichloroethyl octanoate and 14.4 g (450 mmol, 0.9 equivalents) of sulfur Preliminary in 123.0 g of ethanol and heated to an internal temperature of 75° C. under normal pressure. At this temperature, 50.7 g of Na 2 S (650mmol, 1.3 equivalents) of 412.5g aqueous sodium sulfide (12.3% by weight Na in water 2 S) and then continue to stir the reaction mixture at 75°C for 120 min. The reaction contents were cooled to a temperature of 70° C. and then a solution of 94.6 g of sodium borohydride (12% by weight) in sodium hydroxide solution (40% by weight) containing 11.4 g of NaBH was added dropwise uniformly within 30 min. 4 (300 mmol, 0.60 equiv). The ethanol contained was substantially distilled from the reaction mixture over a period of 60 min (up to a distillation head temperature of 98° C.). Then a further 47.3 g of a solution of sodium borohydride (12 wt %) in so...
Embodiment 3
[0065] 123.0 g (500 mmol) of racemic 6,8-dichloroethyl octanoate and 16.0 g (500 mmol, 1.0 equivalent) of sulfur was pre-placed in 123.0 g of ethanol. The autoclave was closed and heated to an internal temperature of 110° C. with stirring. At this temperature, 51.8 g of Na 2S (663 mmol, 1.33 equiv) in 421.0 g of aqueous sodium sulfide (12.3% by weight). Here, the pressure increased from an initial 0.29 MPa to 0.45 MPa. Stirring of the reaction mixture was continued at 110 °C for 120 min, then cooled to 50 °C and transferred to a 2000 ml four-necked flask with stirrer, metering pump, internal thermometer, reflux cooler and oil bath heater. A solution of 99.3 g of sodium borohydride (12% by weight) in sodium hydroxide solution (40% by weight) containing 11.9 g of NaBH was uniformly added dropwise within 30 min at 70°C 4 (315 mmol, 0.63 equiv). The ethanol contained was substantially distilled from the reaction mixture over a period of 60 min (up to a distillation head tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com