Sample diluent, preparation method thereof and immunoassay method for eliminating detection abnormality of fresh sample
A diluent and sample technology, applied in the direction of analysis materials, measuring devices, biological testing, etc., can solve problems such as abnormal test results, reduce non-specific reactions, improve accuracy and precision, and ensure authenticity and reliability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
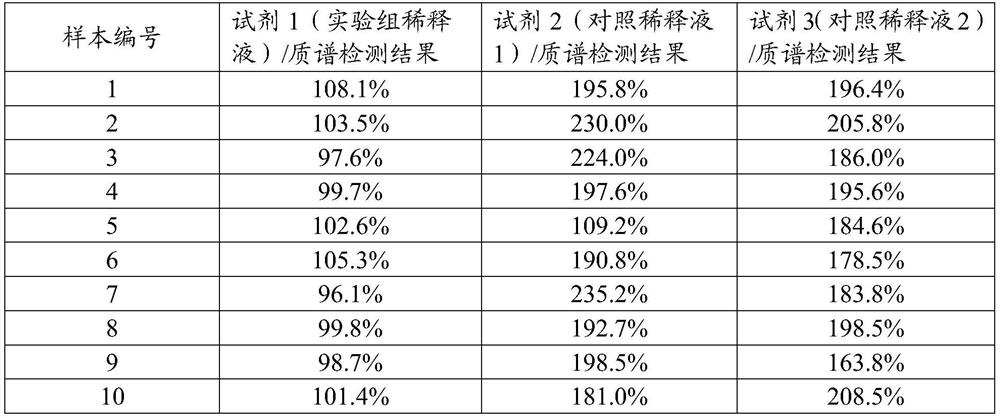
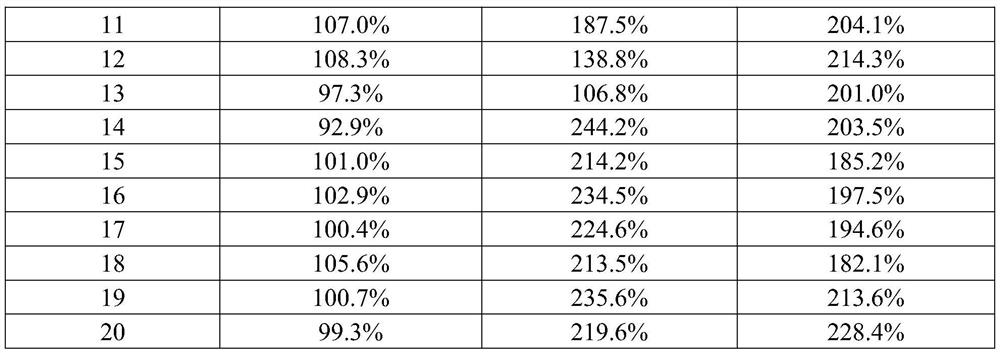
[0031] 1. Diluent formula and preparation method
[0032] Firstly, the diluent of the present invention is prepared, which contains 0.05% lipoic acid, 0.1% dextran sulfate and PBS buffer in mass ratio. Preparation method: First, fully dissolve lipoic acid and dextran sulfate in the above ratio in 0.1mol / L PBS solution, mix well, and then adjust the pH to 7.5 for experimental research.
[0033] The control dilution 1 contains only 0.05% lipoic acid and PBS buffer; the control dilution 2 contains only 0.1% dextran sulfate and PBS buffer. The preparation method of control diluent 1 and control diluent 2 is the same as above.
[0034] 2. Detection method
[0035] The method for detecting the content of 25-hydroxyvitamin D in human serum or plasma samples using the diluent prepared by the present invention specifically comprises the following steps:
[0036] 1. Prepare magnetic particle suspension coated with anti-25 hydroxyvitamin D antibody;
[0037] 2. Preparation of gradien...
Embodiment 2
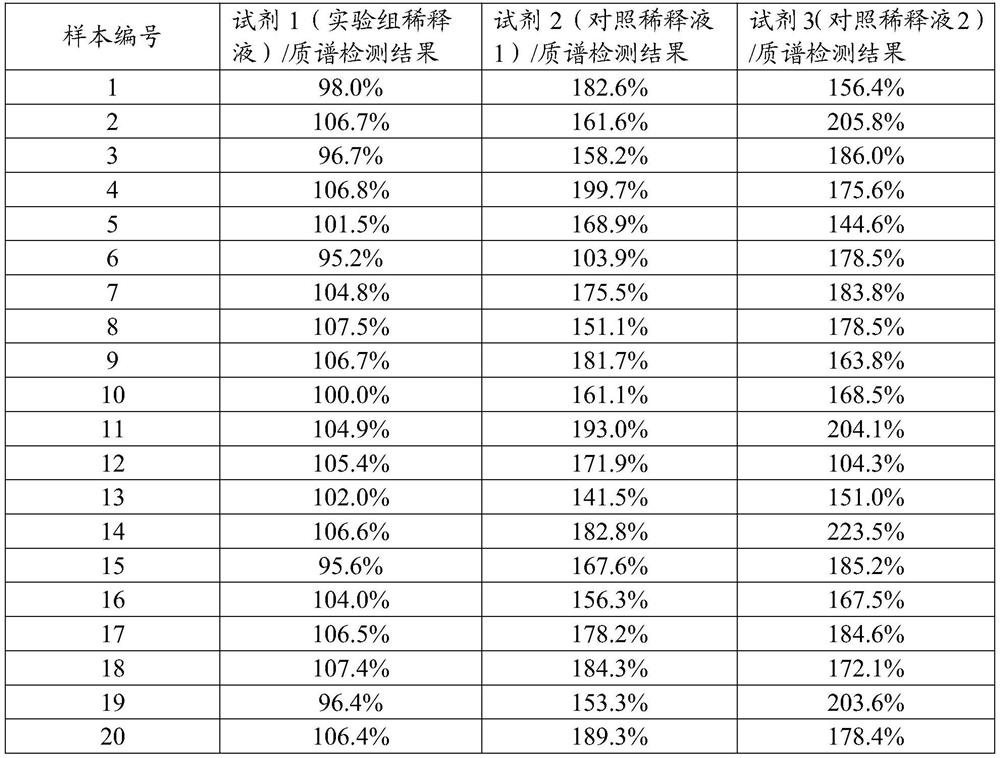
[0051] 1. Diluent formula and preparation method
[0052] Firstly, the buffer solution of the present invention is prepared, which contains 0.5% lipoic acid, 2.5% dextran sulfate and MOPS buffer solution in mass ratio. Preparation method: First, fully dissolve lipoic acid and dextran sulfate in the above ratio in 0.5 mol / L MOPS buffer solution, mix well, and then adjust the pH to 9.0 for experimental research.
[0053] The control dilution 1 contained only 0.5% lipoic acid and MOPS buffer; the control dilution 2 contained only 2.5% dextran sulfate and MOPS buffer. The preparation method of control diluent 1 and control diluent 2 is the same as above.
[0054] 2. Detection method
[0055] The method for detecting the content of trans-triiodothyronine in human serum or plasma samples using the diluent prepared by the present invention specifically comprises the following steps:
[0056] 1. Prepare magnetic particle suspension coated with anti-triiodothyronine antigen;
[005...
Embodiment 3
[0070] 1. Diluent formula and preparation method
[0071] Firstly, the buffer solution of the present invention is prepared, which contains 0.25% lipoic acid, 0.75% dextran sulfate and Tris-HCl buffer solution in mass ratio. Preparation method: First, fully dissolve lipoic acid and dextran sulfate in the above ratio in 0.1mol / L Tris-HCl buffer solution, mix well, and then adjust the pH to 8.5 for experimental research.
[0072] The control dilution 1 is a buffer containing only 0.25% lipoic acid and Tris-HCl; the control dilution 2 is a buffer containing only 0.75% dextran sulfate and Tris-HCl. The preparation method of control diluent 1 and control diluent 2 is the same as above.
[0073] 2. Detection method
[0074] The method for detecting the content of osteocalcin in human serum or plasma samples using the diluent prepared by the present invention specifically comprises the following steps:
[0075] 1. Preparation of magnetic particle suspension coated with anti-osteoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com