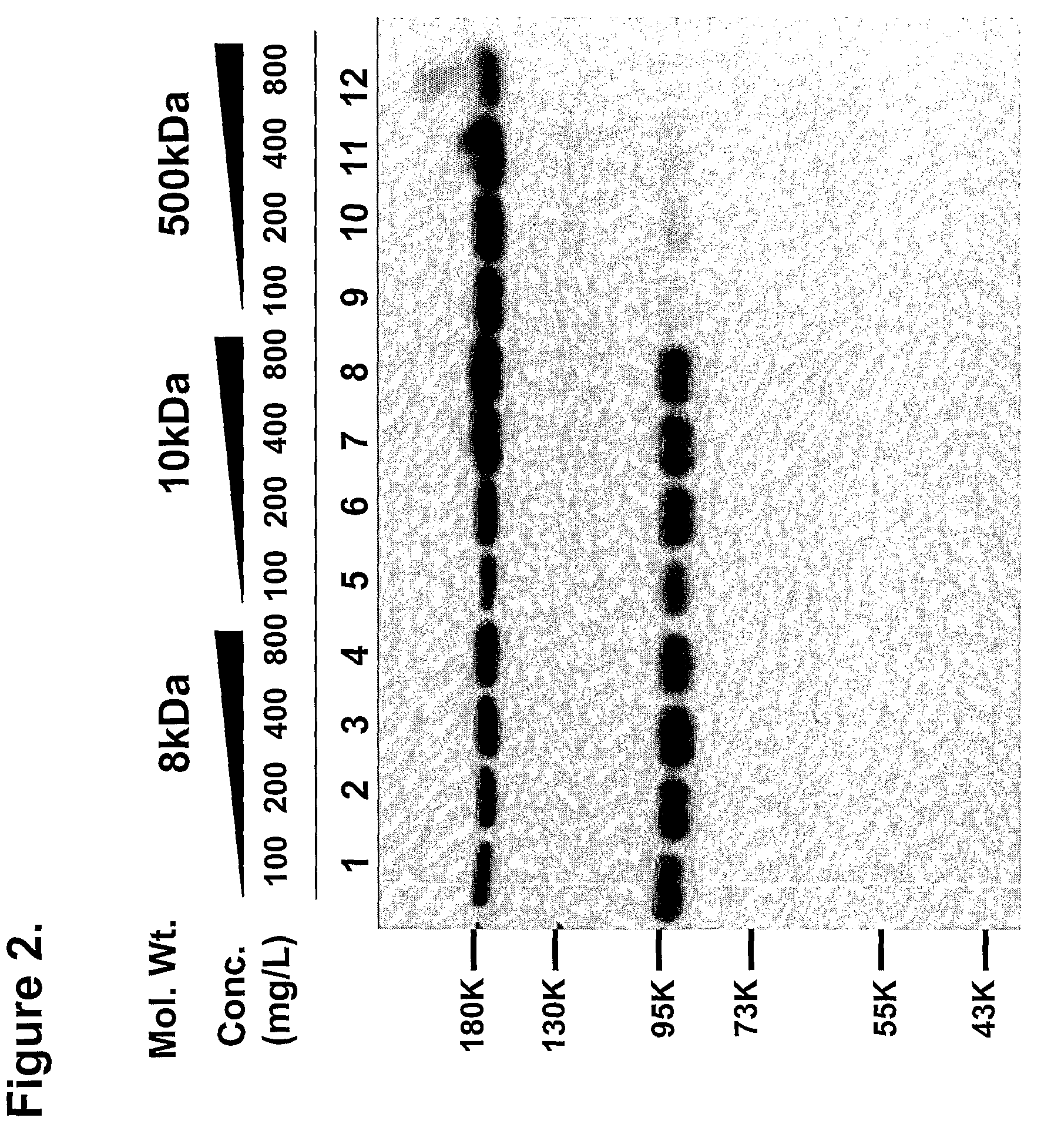
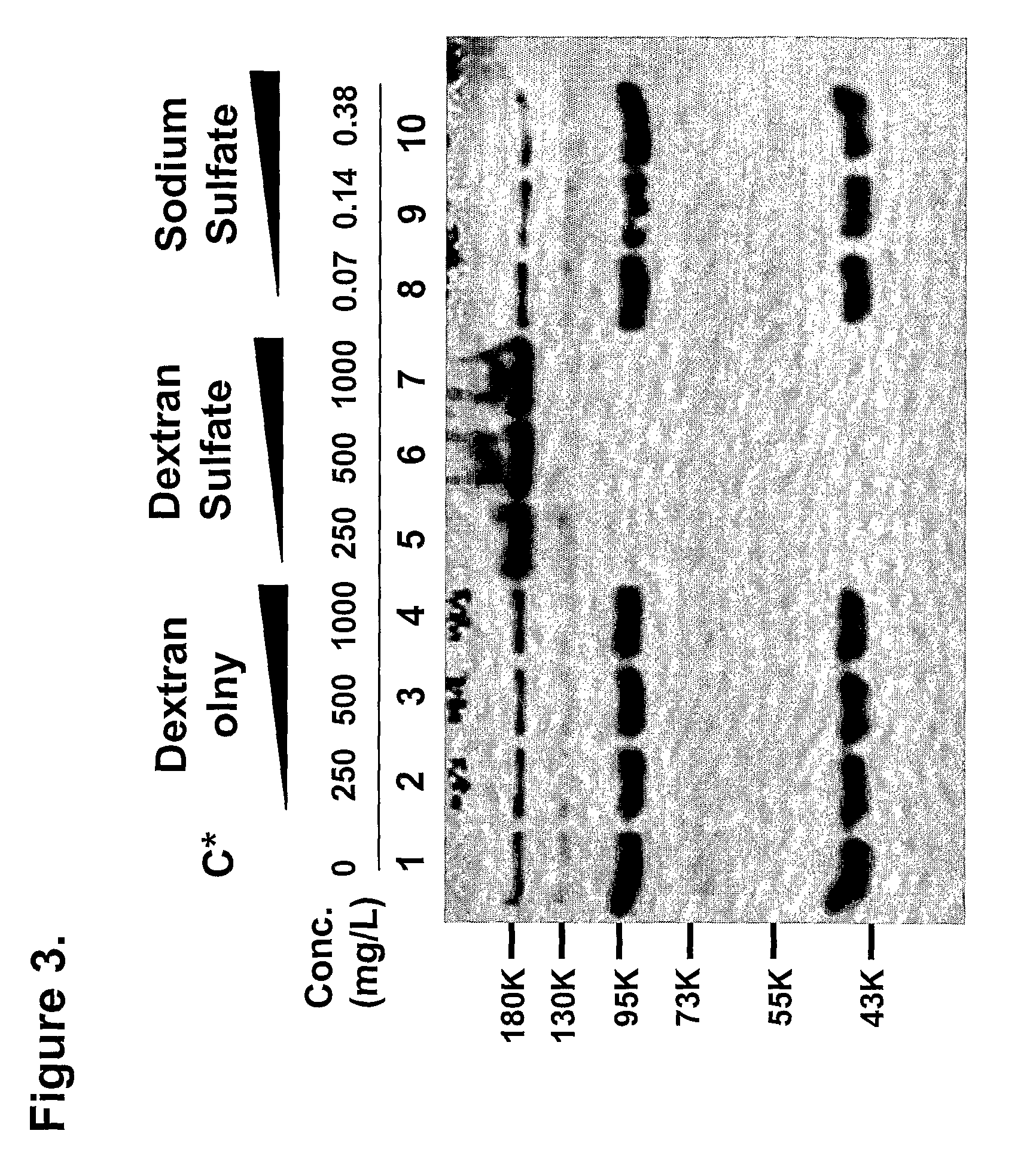
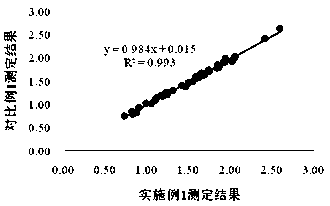
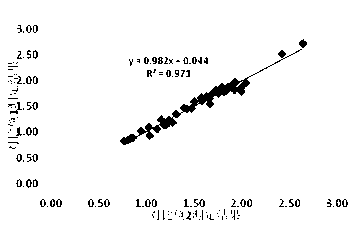
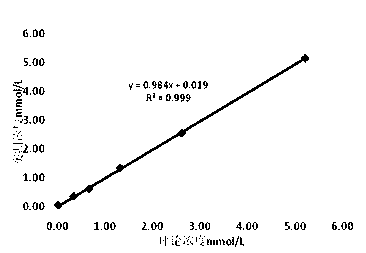
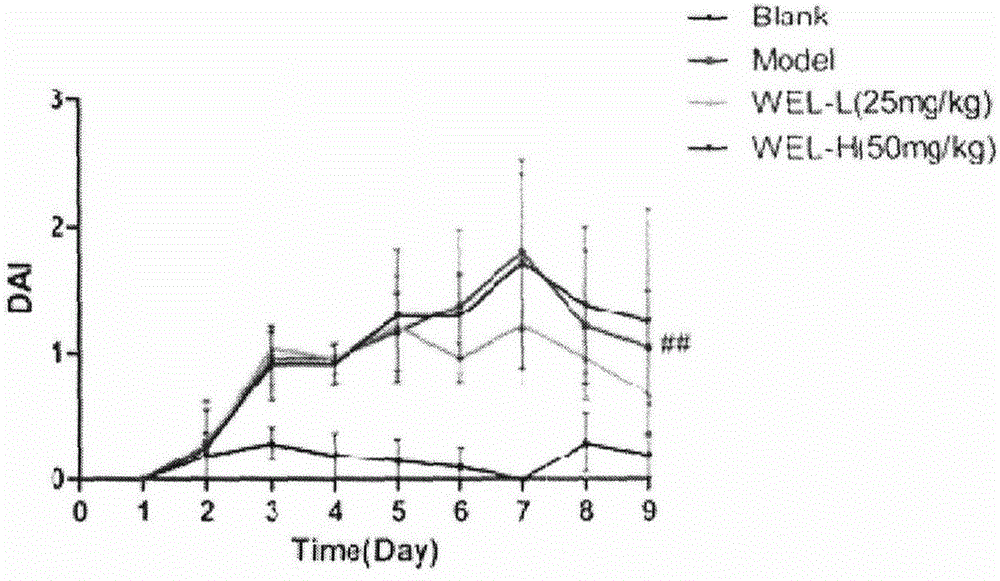
Patents
Literature
74 results about "Dextran sulphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
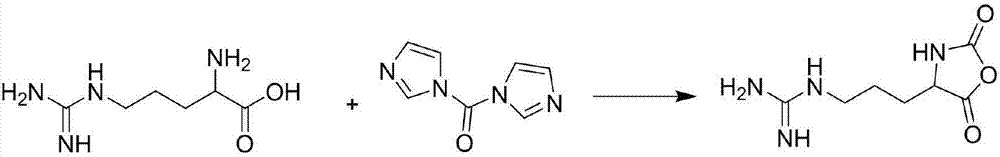
Dextran Sulphate is a polyanionic derivative of Dextran. It is supplied as a white, sodium salt powder, stabilized by a small amount of phosphate.
Methods and compositions based on inhibition of cell invasion and fibrosis by anionic polymers
Owner:TRIAD
Polyesters, method for producing same, and depot medicaments produced from these polyesters
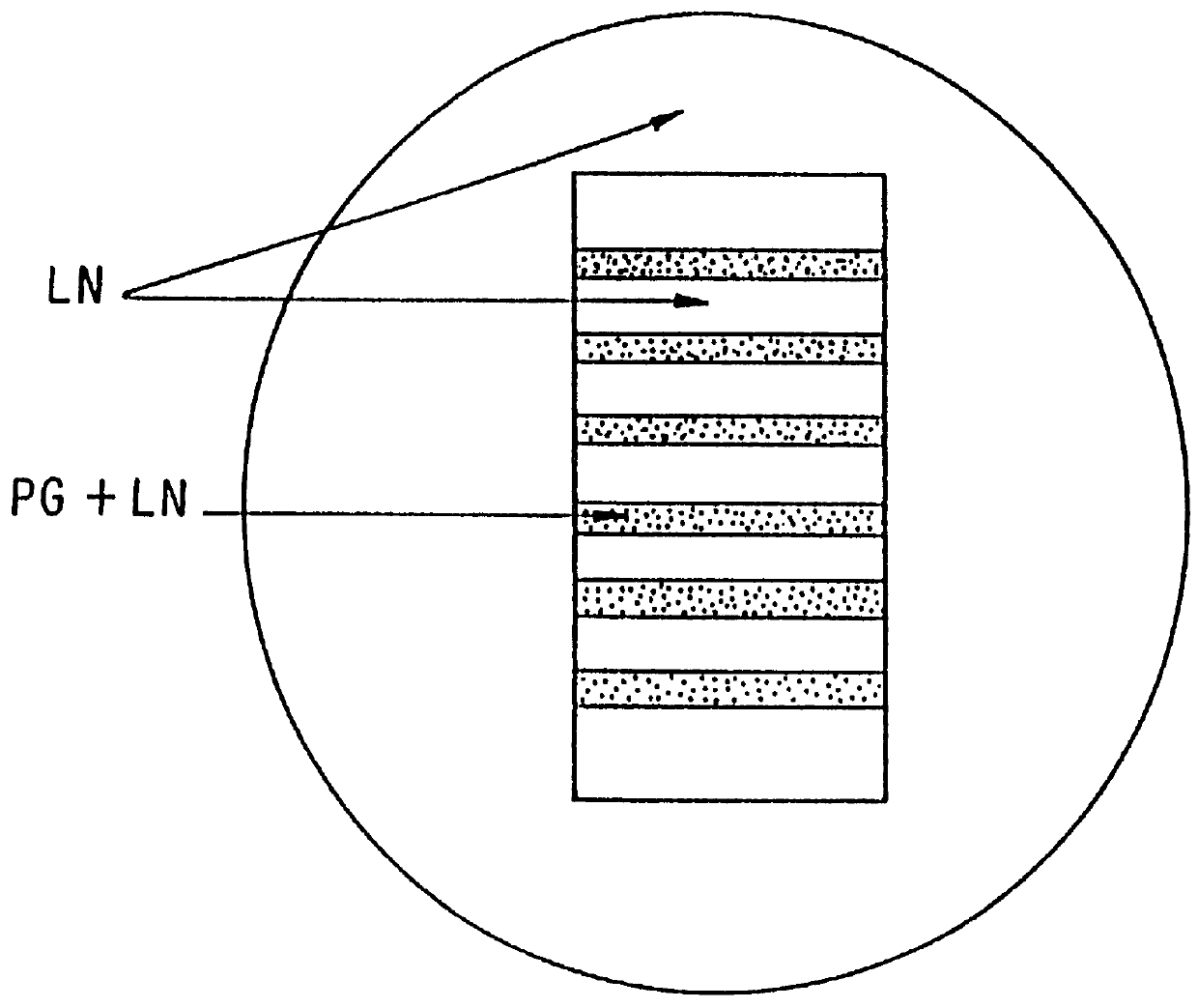
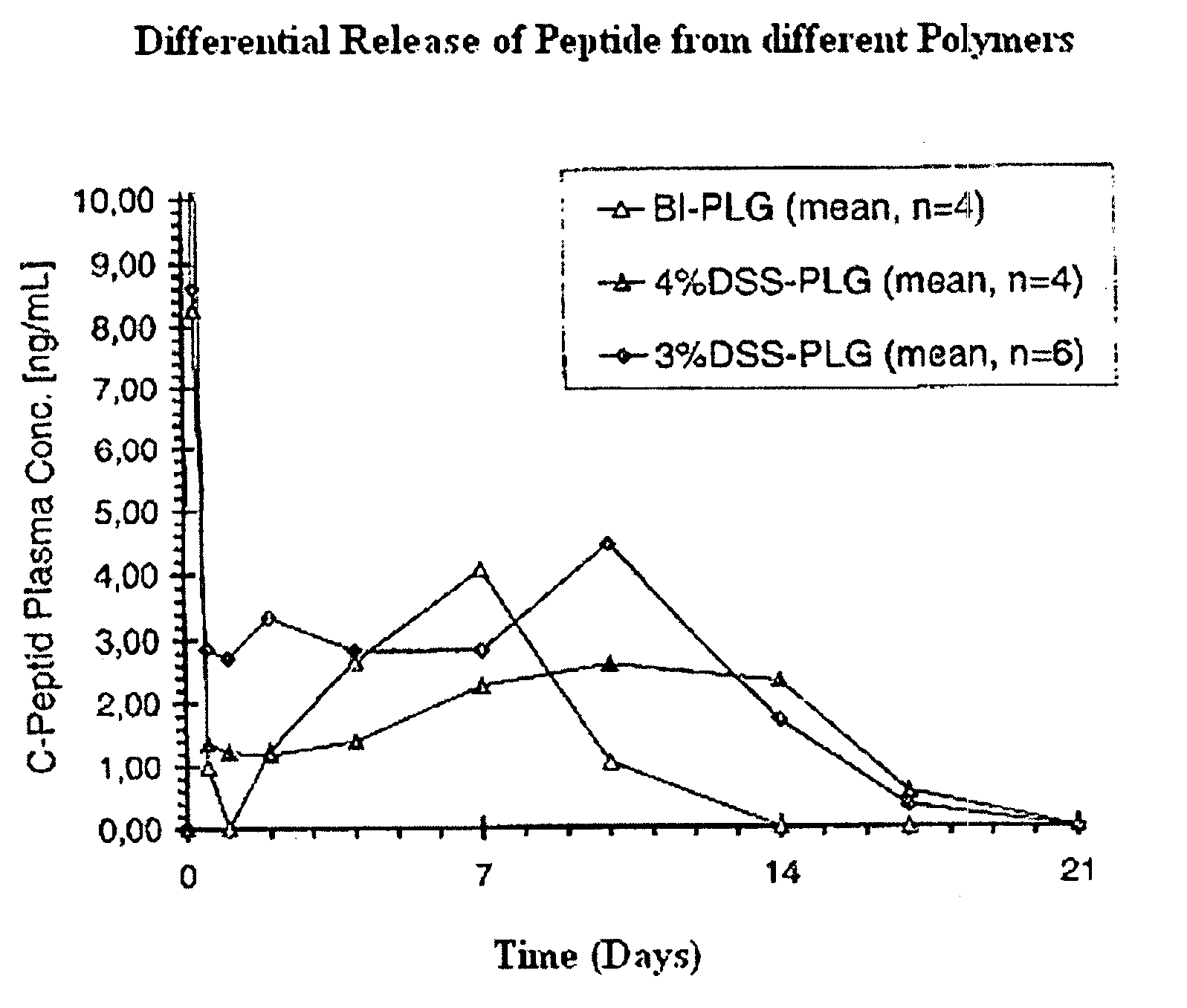
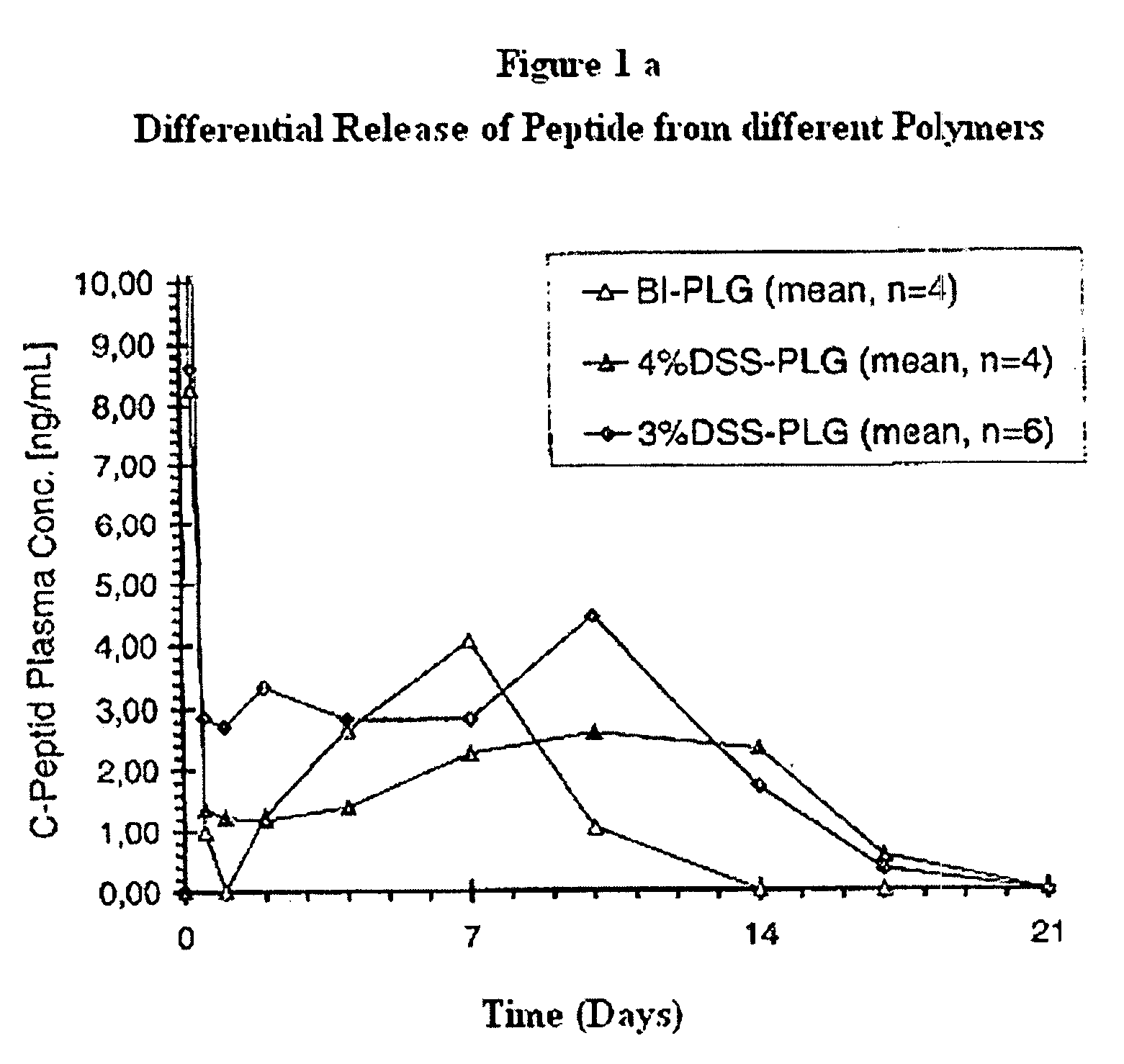
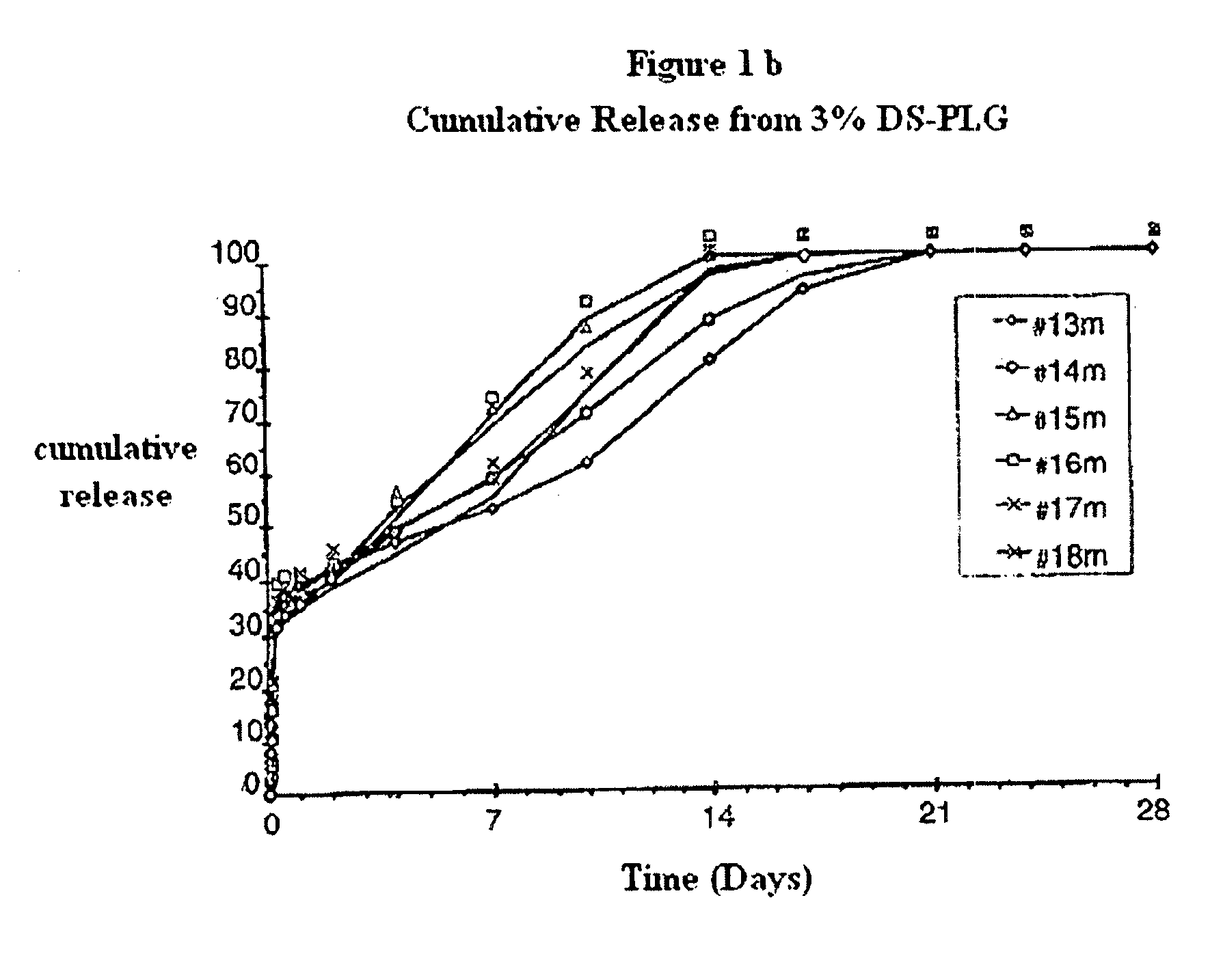
The invention relates to novel absorbable polyesters, produced by ring-opening polymerisation of hydroxycarboxylic acids in the presence of a polyol containing an electrolyte, in an extruder. In particular, the invention relates to novel polylactide glycolide polyesters which are essentially free of dextran sulphate and which are produced by ring-opening polymerisation of lactide and glycolide in the presence of dextran sulphate in the extruder; to the production of the same and to their use in depot medicaments.
Owner:CREATIVE PEPTIDES SWEDEN
Model for protecting mouse ulcerative colitis by antibacterial peptide C-BF
InactiveCN104031883AReduce enzyme activityIncrease secretionTumor/cancer cellsVeterinary instrumentsUlcerative colitisBowels diseases
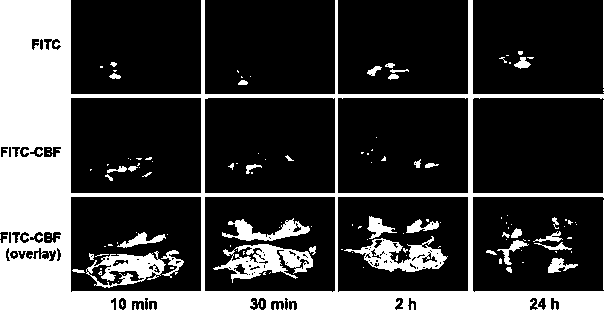
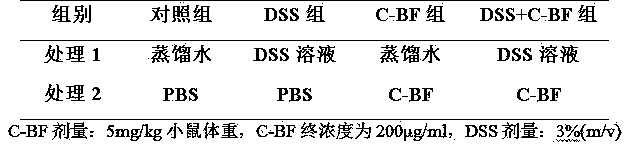
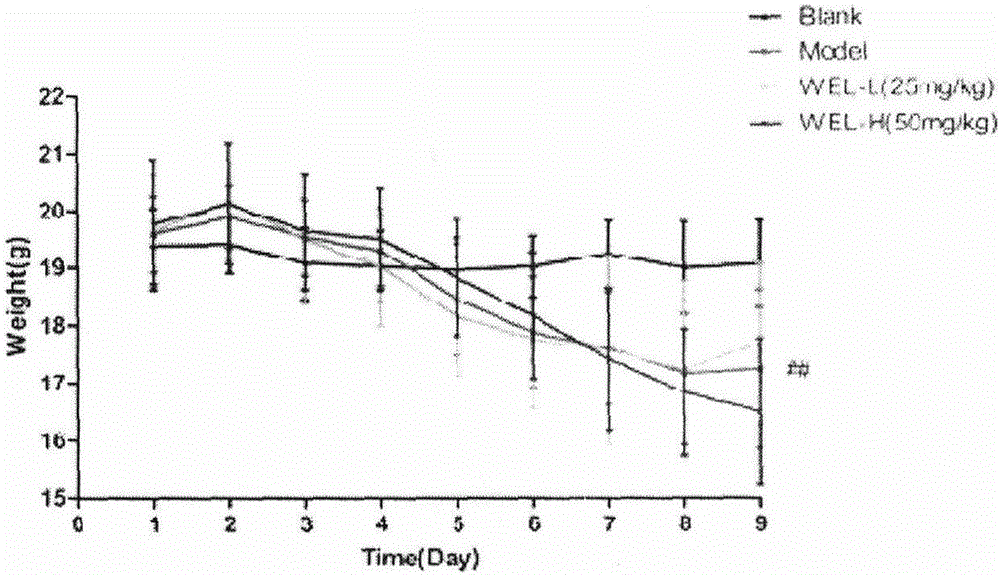
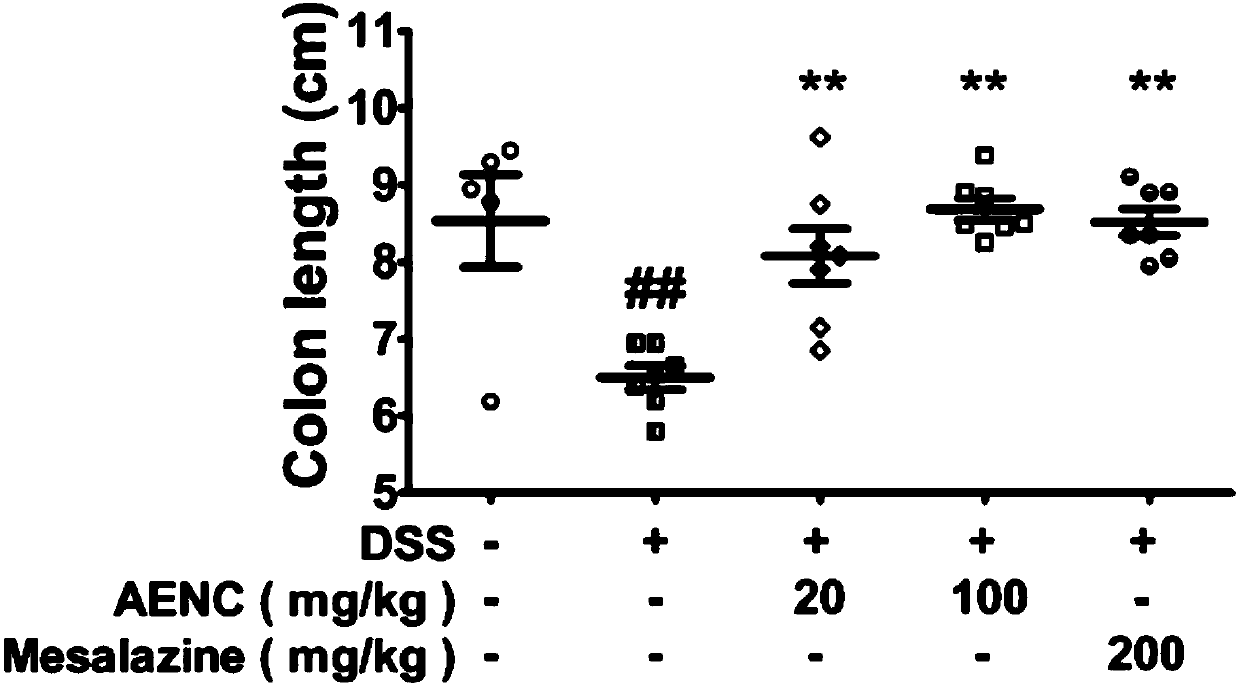
The invention discloses a model for protecting mouse ulcerative colitis by antibacterial peptide C-BF. Cathelicin-BF has broad-spectrum antibacterial activity, especially for Gram negative bacteria. Inflammatory bowel diseases (IBDs) comprise ulcerative colitis (UC) and Crohn disease (CD), and pathomechanisms of the diseases are not clear yet. A model for inducing the mouse ulcerative colitis by 3% dextran sulphate sodium (DSS) is built; a PBS solution of the C-BF is subjected to rectal injection once per day, the dose of the C-BF is 5mg / kg, the continuous injection time is one week, a mouse is weighed and the body weight data of the mouse is recorded each day during experimental period, and the rectal bleeding and dung forming situations of the mouse are observed. The model is used for making a research on a protection effect on the mouse UC caused by the C-BF.
Owner:ZHEJIANG UNIV
Chinese hamster ovary culture medium as well as preparation method and application thereof
InactiveCN102021139ACulture regulationCultivate delivery and controlTissue cultureAdditive ingredientCell culture media
The invention discloses a Chinese hamster ovary culture medium as well as a preparation method and application thereof. The culture medium comprises ascorbic acid, pyridoxine hydrochloride, vitamin B12, nicotinamide, riboflavin, thiamine chloride, choline chloride, folic acid, calcium pantothenate, sodium pyruvate, reduced glutathione, inositol, biotin, hypoxanthine, putrescine, lipoic acid, linoleic acid, thymine, glucose, HEPES (2-hydroxyethyl) buffer solution, dextran sulphate, Pluronic-F68, various amino acids and salts thereof and various inorganic salts. The culture medium has low cost, and the component does not contain animal origin ingredients, does not contain protein and has definite chemical ingredients. All ingredients are cooperated and blended, and the Chinese hamster ovary culture medium can satisfy the basic growth requirement of cells, and can effectively regulate, transfer and control the culture of cells so as to realize good high-density cell culture. Compared with the culture effect of the existing culture medium, the culture effect of culture medium disclosed in the invention is at least equivalent to even superior to the existing culture medium.
Owner:EAST CHINA UNIV OF SCI & TECH
Application of dextran sulfate in preparing medicament for treating hepatic fibrosis
InactiveCN102973593ARelieve symptomsStrong inhibition of activationOrganic active ingredientsRespiratory disorderPharmaceutical drugCurative effect
The invention discloses novel application of dextran sulfate in preparing a medicament for treating hepatic fibrosis. The medicament can be used for inhibiting inflammatory reaction in hepatic fibrosis attaching process, so that attack or symptom worsening of hepatic fibrosis can be inhibited. Dextran sulfate has good curative effect in a carbon tetrachloride induced animal model; and the medicaments used in the application can be easily acquired, low in price and stable in properties, are convenient to store and transport, and have wide application prospect.
Owner:合肥博太医药生物技术发展有限公司
Preparation method of guanidinated chitosan and application of guanidinated chitosan
ActiveCN106905443AImprove the grafting rate of guanidinylationLow toxicityBiocideFungicidesSolubilityMicrosphere
The invention provides a preparation method of guanidinated chitosan. By guanidination modification for chitosan, the biological alkalinity and the water solubility of the chitosan are improved, and the action capacity of the chitosan to an electronegative cell membrane is enhanced; the provided preparation method of the guanidinated chitosan aims to increase the grafting rate of chitosan guanidination and avoid side reaction. The invention further provides a method for preparing antibacterial nano microspheres from the guanidinated chitosan. The method comprises the steps of by taking dextran sulfate as a crosslinking agent, preparing the lysozyme-coated guanidinated chitosan nano microspheres; by the use of the puncture action of the guanidinated chitosan to the cell membrane, the stability of the outer membrane lipid structure of gram negative bacteria (G-) is destroyed to expose peptidoglycan on the inner wall layer, and the peptidoglycan is hydrolyzed through lysozyme; under the synergistic action of the guanidinated chitosan and the lysozyme-coated guanidinated chitosan nano microspheres, the inhibition effect on G- is enhanced.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
BMP-2 (Bone Morphogenetic Protein-2) sustained release microsphere and preparation method thereof
InactiveCN103169950AEvenly distributedEasy to shapePeptide/protein ingredientsSkeletal disorderMicrosphereFreeze-drying
The invention discloses a BMP-2 (Bone Morphogenetic Protein-2) sustained release microsphere and a preparation method thereof. The preparation method of the BMP-2 sustained release microsphere comprises the following steps of: dissolving chitosan in an aqueous solvent so as to obtain a chitosan solution; dissolving BMP-2 into a dextran sulfate sodium aqueous solution, dropwise adding the chitosan solution into the dextran sulfate sodium aqueous solution under the condition of stirring, then adding a metal salt so as to form the microsphere in a reaction solution, washing and freeze-drying so as to obtain the BMP-2 sustained release microsphere. The matrix component of the microsphere prepared by using the method comprises chitosan and dextran sulfate sodium, the wrapped medicine is BMP-2, the average particle size of the microsphere is 200-900nm, the encapsulation efficiency can be 75-90%, and the BMP-2 is long in protein activity duration and sustained release period. The BMP-2 sustained release microsphere is simple in preparation process, low in production cost, easy to carry out, and mild in reaction condition, and does not need the addition of reagents which can influence the protein activity of BMP-2; and the e microsphere is round and complete in appearance, good in polydispersity, non-sticky after being freeze-dried and soluble in water.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Method for building dextran sodium sulphate (DSS) induced enteritis mouse model
ActiveCN102813671AReduce mortalityRepeatableOrganic active ingredientsIn-vivo testing preparationsMortality rateDextran
The invention provides a method for building a dextran sodium sulphate induced enteritis mouse model. The method includes the following steps: A, choosing female C57BL / 6 mice with the weight of 19-21g as the objects to build the mouse model; B, dissolving dextran sulphate sodium salt solution in disinfected drinking water to prepare dextran sulphate sodium salt solution with the weight percentage concentration of 2%, and placing the solution in a water bottle for protecting away from the sun, wherein the solution is prepared when required; C, feeding the chosen C57BL / 6 mice in a storageprotect feature (SPF) level animal house, enabling the mice to drink the dextran sulphate sodium salt solution prepared in the step B for six days and then drink the normal drinking water for four days. Accordingly, the chosen C57BL / 6 mice are dextran sodium sulphate induced enteritis mouse models. By means of the method for building the dextran sodium sulphate induced enteritis mouse model, death rate of animals is reduced to be zero, and pathology expressions are the same, so that the model is stable, reliable and repeatable.
Owner:辉源生物科技(上海)有限公司
Process for producing and purifying factor VIII and its derivatives
ActiveUS9441030B2Reducing and blocking its activityImprove homogeneityFactor VIIPeptide preparation methodsProtein targetCultured cell
Disclosed is a method for producing proteins having factor VIII procoagulant activity in serum-free medium by in vitro culturing of mammalian cells, wherein the serum-free medium contains an inhibitor against the protease released from cultured cells. In accordance with this invention, the inhibitor can protect the cleavage of a target protein during cultivation and increase homogeneity of a target molecule, wherein the inhibitor can be a dextran sulfate. This invention also relates to a method of purifying target molecules from the culture medium containing both a target molecule and selected inhibitors by affinity chromatography.
Owner:SK BIOSCI CO LTD
Recombinant bacillus subtilis expressing C30 carotenoid
ActiveCN105838731ARelieves Colitis SymptomsBacteriaMicrobiological testing/measurementDendritic cellGenetic engineering
The invention relates to recombinant bacillus subtilis capable of synthesizing carotenoid 4,4'-diaponeurosporene with 30 carbon atoms (C30), and belongs to the biotechnology genetic engineering field. A bacillus subtilis expression plasmid pMK3-crtmn (which is capable of expressing C30 carotenoid synthases Crtm and Crtn in bacillus subtilis) containing C30 carotenoid synthase genes crtm and crtn is successfully constructed, and is successfully electro-transformed into bacillus subtilis WB800. The WB800 strain after transformation turns from the original color of white to yellow. A pigment component extracted from the recombinant WB800 is identified as 4,4'-diaponeurosporene by high performance liquid chromatography (HPLC). 4,4'-diaponeurosporene can stimulate maturation of dendritic cells, and generated cytokines (IL-6, IL-10, IL-12p70 and TNF[alpha]) have the amount increased, and moreover and have stronger ability to stimulate proliferation of T lymphocytes. With oral administration of the bacillus subtilis capable of synthesizing 4,4'-diaponeurosporene, the dextran sulphate sodium (DSS)-induced mice colitis symptoms can be significantly reduced. The bacillus subtilis capable of producing 4,4'-diaponeurosporene is expected to be developed as probiotics for prevention of colitis.
Owner:NANJING AGRICULTURAL UNIVERSITY
Binding of pathological forms of prion proteins
Infective aggregating forms of proteins such as PrP, amyloid, and tau are bound selectively in the presence of the normal form protein using a polyionic binding agent such as dextran sulphate or pentosan (anionic), or polyamine compounds such as pDADMAC (cationic) under selective binding conditions including the use of n-lauroylsarcosine at mildly alkaline pH, and may then be assayed.
Owner:MICROSENS BIOPHAGE LTD
Recombinant human lactoferrin (rhLF) silkworm chrysalis powder as well as preparation method and application thereof
InactiveCN104561098AImprove biological activitySuitable for mass productionPeptide/protein ingredientsMicroencapsulation basedFreeze-dryingUlcerative colitis
The invention relates to recombinant human lactoferrin (rhLF) silkworm chrysalis powder as well as a preparation method and application thereof and belongs to the technical field of biological medicines. A recombinant virus is constructed mainly by adopting a silkworm chrysalis baculovirus expression system, the rhLF is expressed with a silkworm chrysalis serving as a bioreactor, the silkworm chrysalis diseased after being inoculated with the recombinant virus is prepared into freeze-dried powder, the activity of the freeze-dried powder is detected by performing antibacterial experiments in vitro, and the freeze-dried powder is poured into the stomach of a mouse to treat the dextran sulphate sodium (DSS) induced ulcerative colitis of the mouse. The analysis on the disease activity index (DAI) of the mouse and the detection on the content of myeloperoxidase (MPO) in the colonic tissue of the mouse show that the rhLF with antibacterial activity is expressed successfully in the silkworm chrysalis, and the rhLF silkworm chrysalis powder has a certain effect of treating the DSS induced ulcerative colitis of the mouse. The preparation method provided by the invention is simple, is low in cost and is used for preparing a novel oral protein medicine.
Owner:TIANJIN YAOYU BIOLOGICAL TECH
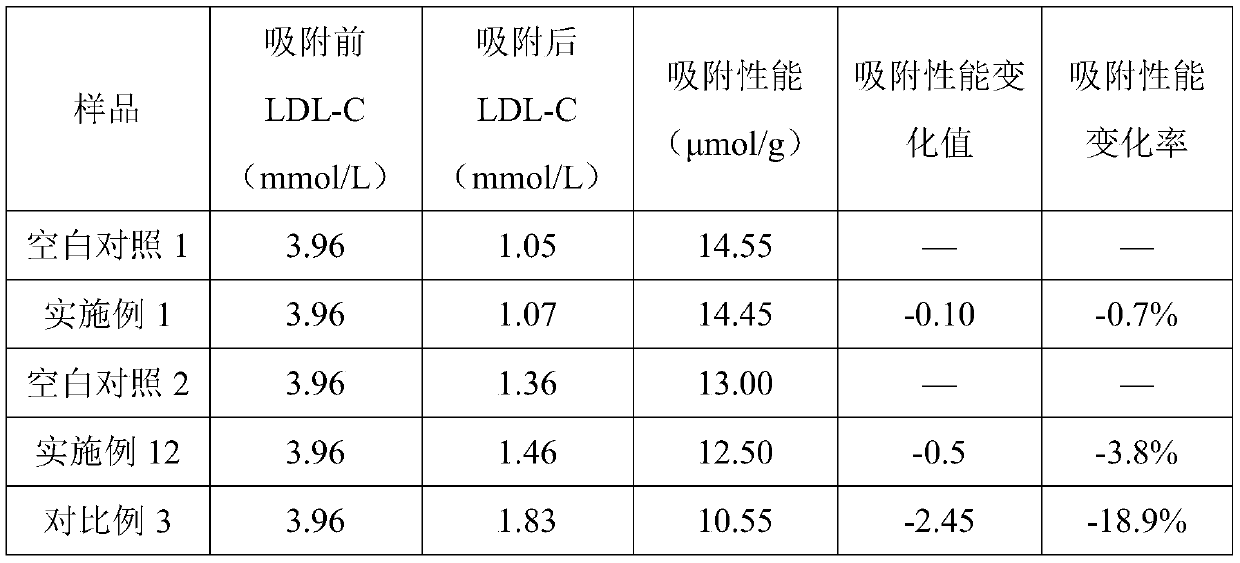
Adsorbent for extracorporeal blood circulation to remove LDL, preparation method thereof, and perfusion device
ActiveCN109248668AEnsure safetyImprove bindingOther chemical processesSuction devicesSpecific adsorptionExtracorporeal circulation
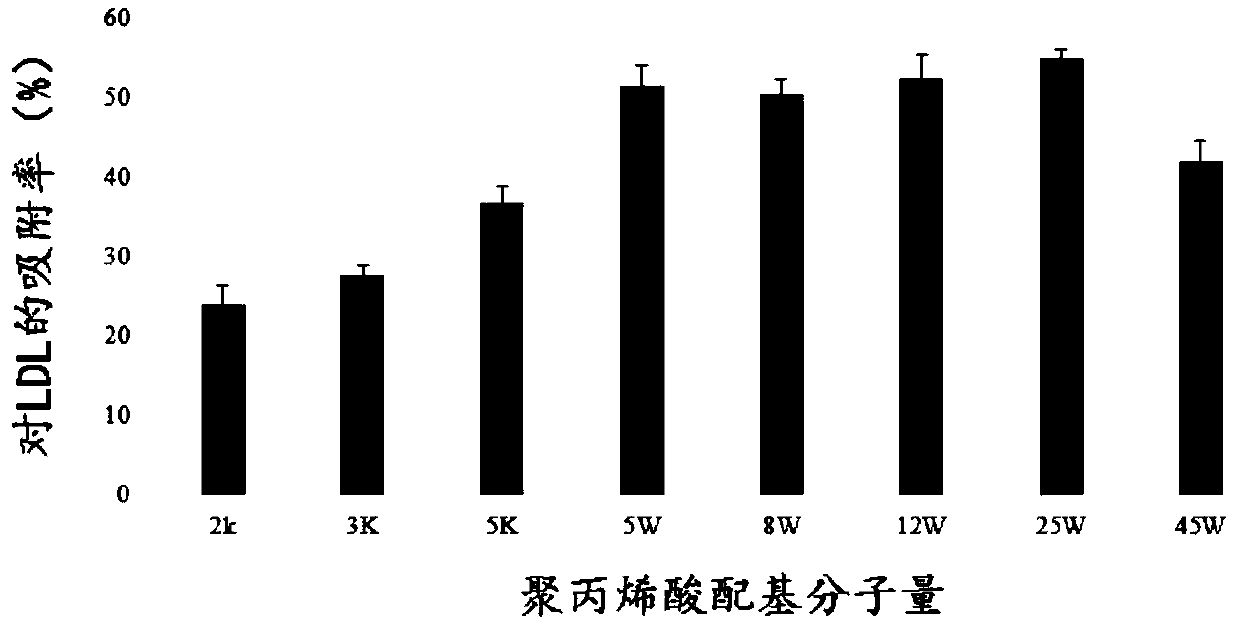
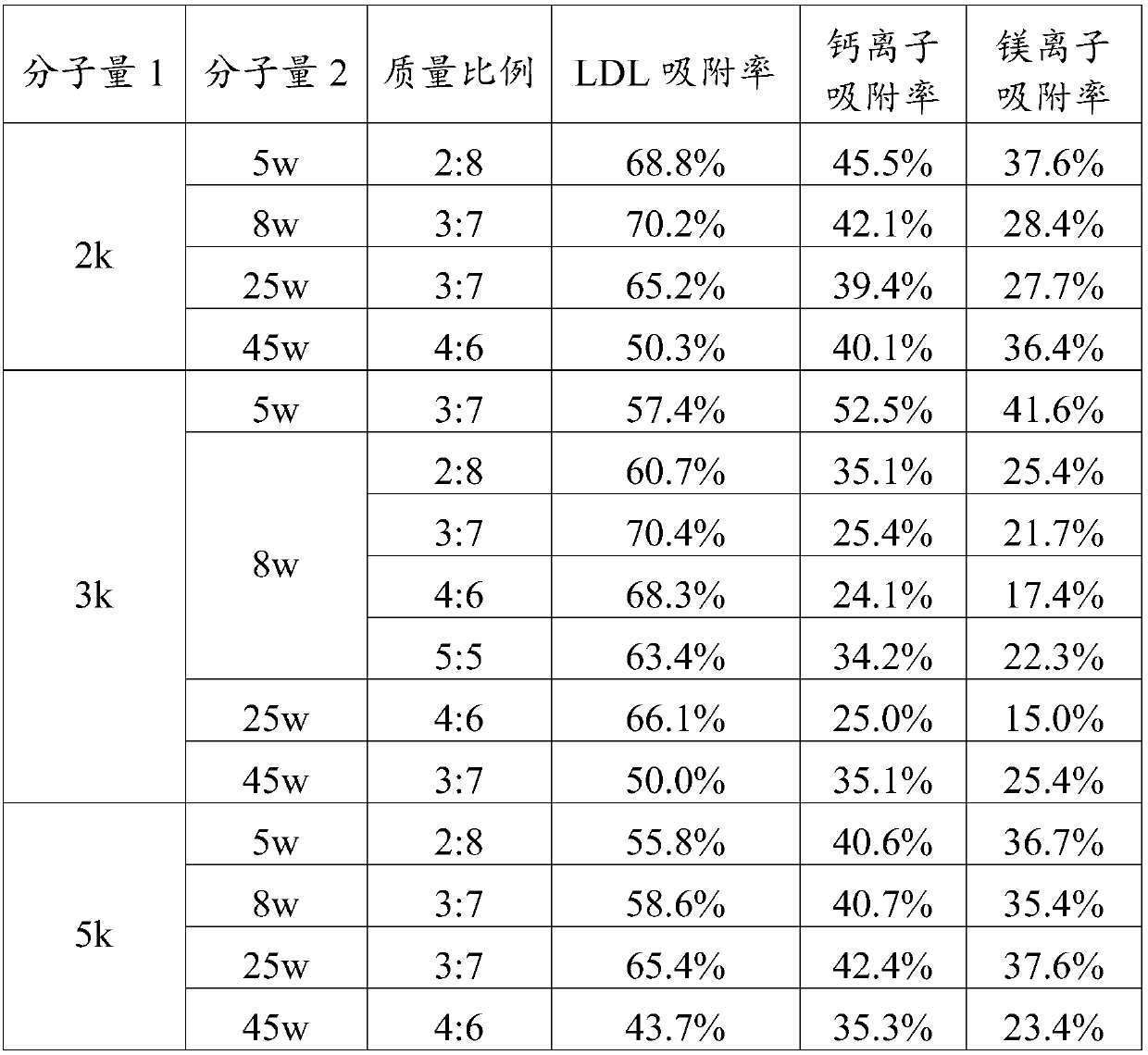
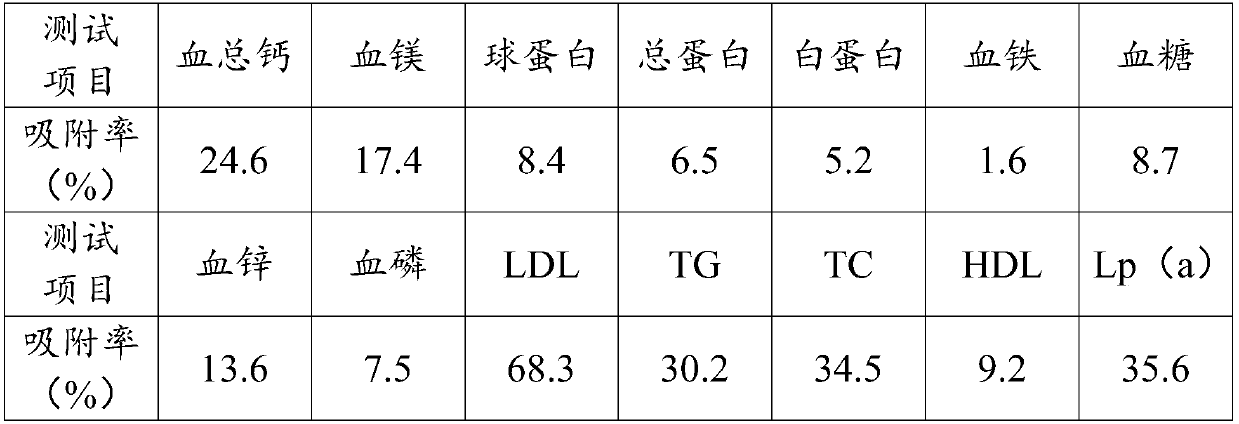
The invention relates to an adsorbent for extracorporeal blood circulation to remove LDL, a preparation method thereof, and a perfusion device. The adsorbent is formed by introducing an amino group toa carrier and reacting the amino group with a polyanion; and the polyanion is at least one of polyacrylic acid, carbomer and dextran sulfate, wherein the polyacrylic acid is a polyacrylic acid havinga molecular weight of 5,000 to 450,000, or the polyacrylic acid is a mixture of at least two polyacrylic acids with different molecular weights; the carbomer is carbomer with a single grade, or the carbomer is a mixture of at least two carbomers with different grades; and the dextran sulfate is subjected to an hydroformylation reaction together with periodate prior before reacting with the aminogroup, and is subjected to a reduction reaction after reacting with the amino group. The adsorbent has a high adsorption rate and specific adsorption to the LDL.
Owner:JAFRON BIOMEDICAL
Serum high-density liptein cholesterol test kit
ActiveCN111534568ANo reconstitution requiredImprove stabilityMicrobiological testing/measurementMethylanilineAmpyrone
The invention provides a high-density liptein cholesterol test kit. The kit contains a reagent R1 and a reagent R2; the reagent R1 contains the following components: 3-morpholinopropanesulfonic acid (MOPS) buffer solution, alpha-cyclic glucan sulfate, dextran sulfate, pulullan, magnesium chloride, N-ethyl-N-(2-hydroxyl-3-sulfopropyl)-3-sodium methylaniline, a surface active agent, and a preservative; and the reagent R2 contains the following components: 3-morpholinopropanesulfonic acid (MOPS) buffer solution, cholesterol esterase (CEH), cholesterol oxidase (COD), peroxidase (POD), 4-ampyrone,flavin adenine dinucleotide disodium, a surface active agent, a stabilizer, and a preservative. The invention also provides a preparation method and application of the kit. The kit is a liquid kit, which is high in stability, anti-interference capability and accuracy, and good in repeatability.
Owner:中拓生物有限公司 +2
Application of wedelolactone in preparing drug for resisting ulcerative colitis
InactiveCN105030763AImprove colitis symptomsGood treatment effectDigestive systemHeterocyclic compound active ingredientsInflammatory factorsWedelolactone
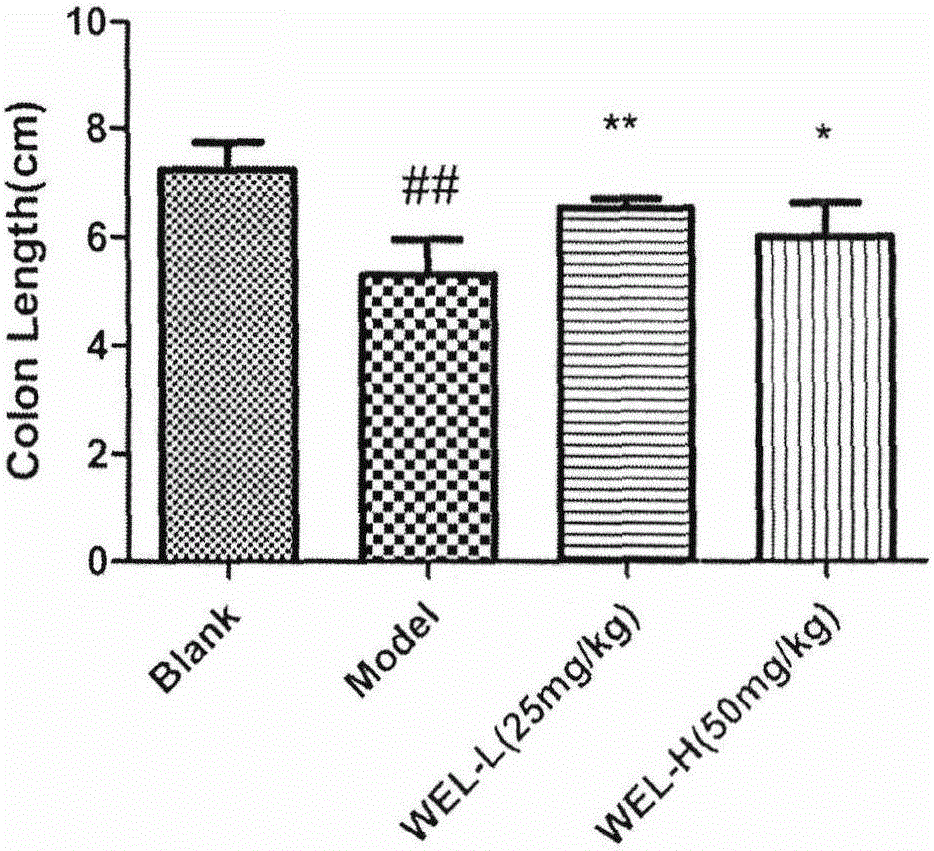
The invention relates to an application of Chinese traditional herb monomer wedelolactone as a drug for treating ulcerative colitis. According to the application, wedelolactone is obtained from Chinese traditional herb material eclipta, and the structure of wedelolactone is determined according to spectrum data. Through intragastric administration of wedelolactone, the body weight change of a model mouse is obviously changed, the length of the colon is increased, the inflammation level of the colon is obviously hanged, the NO content reflecting the inflammation degree of the colon is reduced, the activity of myeloperoxidase (MPO) in the colon tissue is reduced, release of inflammatory factors IL-8 of Caco-2 cells excited byIL-1beta is inhibited in vitro. The results of in-vivo and in-vitro experiments show that wedelolactone under certain dosage can obviously inhibit release of the inflammatory factors of the colon tissue, so that the acute ulcerative colitis of the mouse caused by dextran sulphate sodium salt (DSS) can be obviously improved, and therefore, wedelolactone has a novel application as a drug for treating or improving ulcerative colitis.
Owner:CHINA PHARM UNIV
Cellulose sulfate and other sulfated polysaccharides to prevent and treat papilloma virus infection and other infections
A method for treating and preventing various infections, including papilloma virus and fungal and parasitic infections is provided. In particular, an effective amount of a sulphated polysaccharide, such as cellulose sulphate and dextran sulphate are administered to prevent and treat these infections. The invention also relates to use of these compounds for the prevention and inhibition of malignant epithelial lesions associated with papilloma virus, such as cervical cancer.
Owner:RUSH PRESBYTERIAN ST LUKES MEDICAL CENT +1
Salvianic acid A sodium self-assembled liposome for treating ulcerative colitis and preparation method thereof
PendingCN113398073AImprove clinical symptomsIncrease TNF-αOrganic active ingredientsAntipyreticInflammatory factorsCholesterol
The invention belongs to the technical field of medicines, and particularly relates to a salvianic acid A sodium oral self-assembled liposome for treating ulcerative colitis and a preparation method thereof. The salvianic acid A sodium oral self-assembled liposome for treating ulcerative colitis prepared by the method disclosed by the invention is prepared by taking salvianic acid A sodium, phospholipid, cholesterol, chitosan and sodium alginate as raw materials and adopting a reversed-phase evaporation method and an LbL self-assembly technology. A mouse experiment of the DSS (dextran sulfate sodium) induced ulcerative colitis proves that the oral salvianic acid A sodium self-assembled liposome prepared by the invention has the effects of improving clinical symptoms of mice with colitis, reducing the content of proinflammatory factors TNF-alpha, IF-6 and IF-1beta in serum of the mice with ulcerative colitis, increasing the content of anti-inflammatory factor IL-10 and alleviating intestinal flora imbalance of mice with ulcerative colitis, thereby having a relatively good medicine effect on treating ulcerative colitis.
Owner:ZHEJIANG UNIV OF TECH
Medium supplements for improved process performance
The present invention pertains to a cell culture medium comprising dextran sulfate or a mixture of dextran sulfate and ferric citrate, and methods of using thereof. The present invention further pertains to a method of producing a protein of interest in a large scale cell culture, comprising supplementing the cell culture with dextran sulfate or a mixture of dextran sulfate and ferric citrate.
Owner:GLORIANA THERAPEUTICS INC
Application of caffeic acid 3,4-dyhydroxy phenethyl ester to preparing medicine for preventing colorectal cancer
InactiveCN108992434APrevent intrusionPrevent infiltrationOrganic active ingredientsAntineoplastic agentsDimethylhydrazineCyclin D1
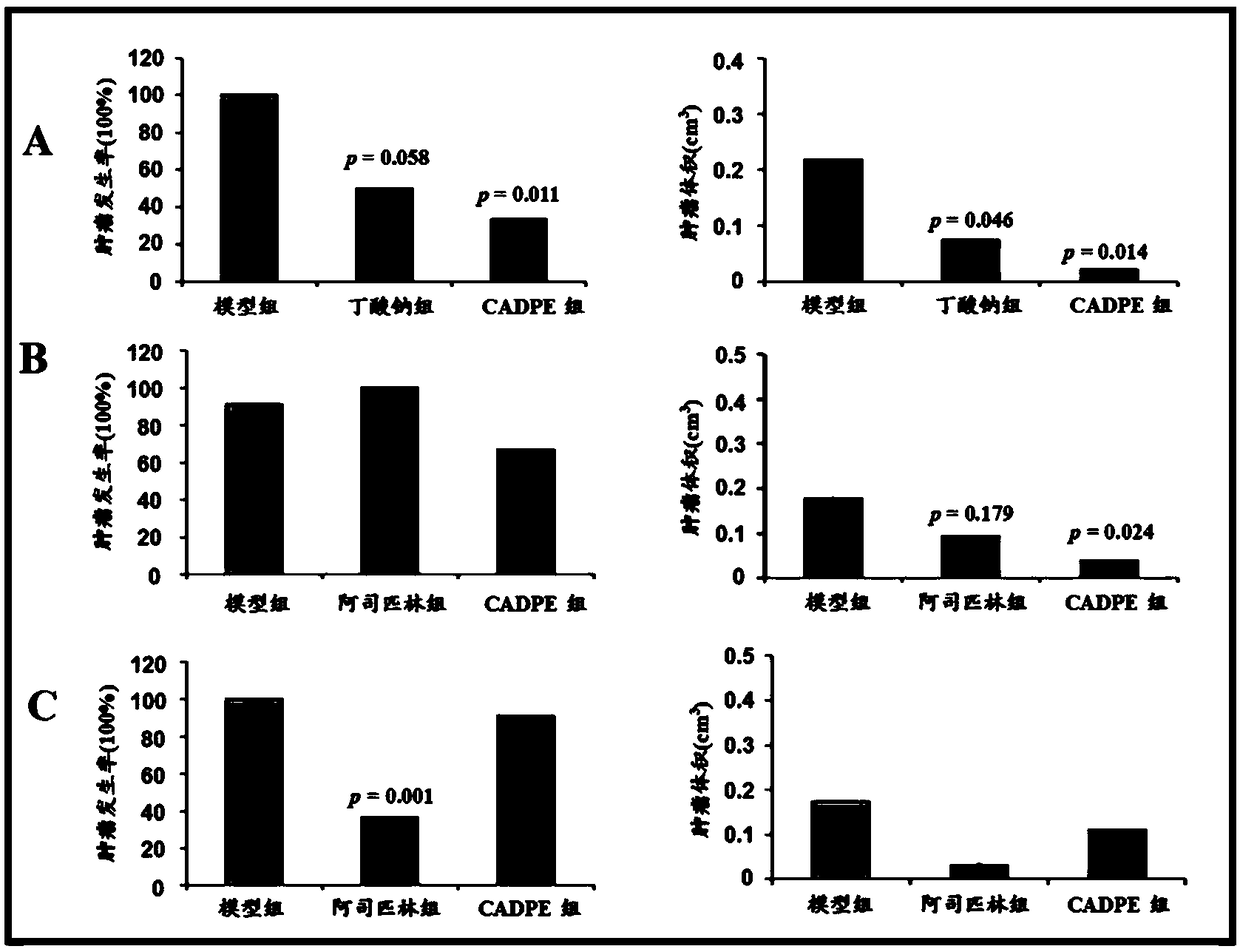
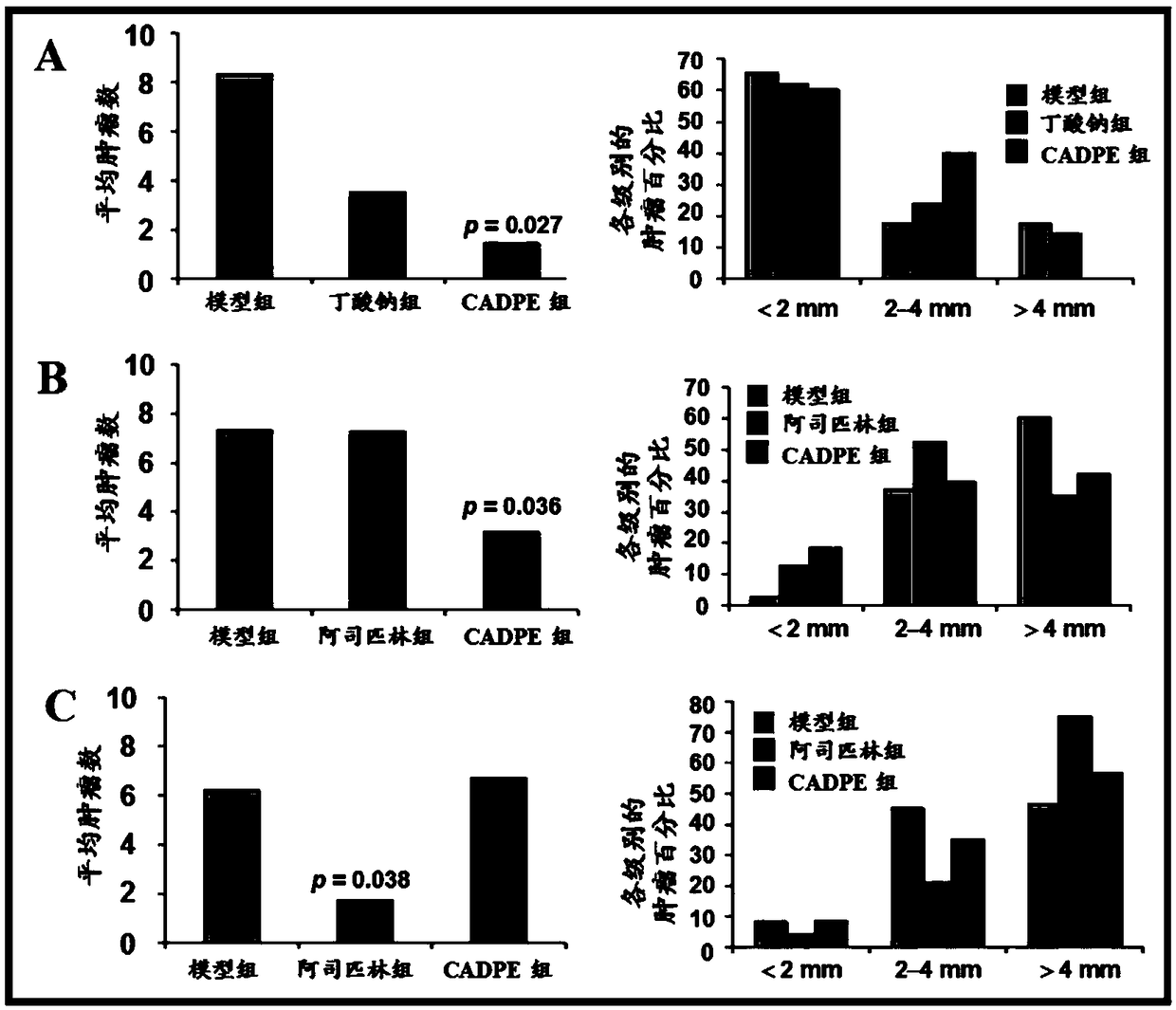
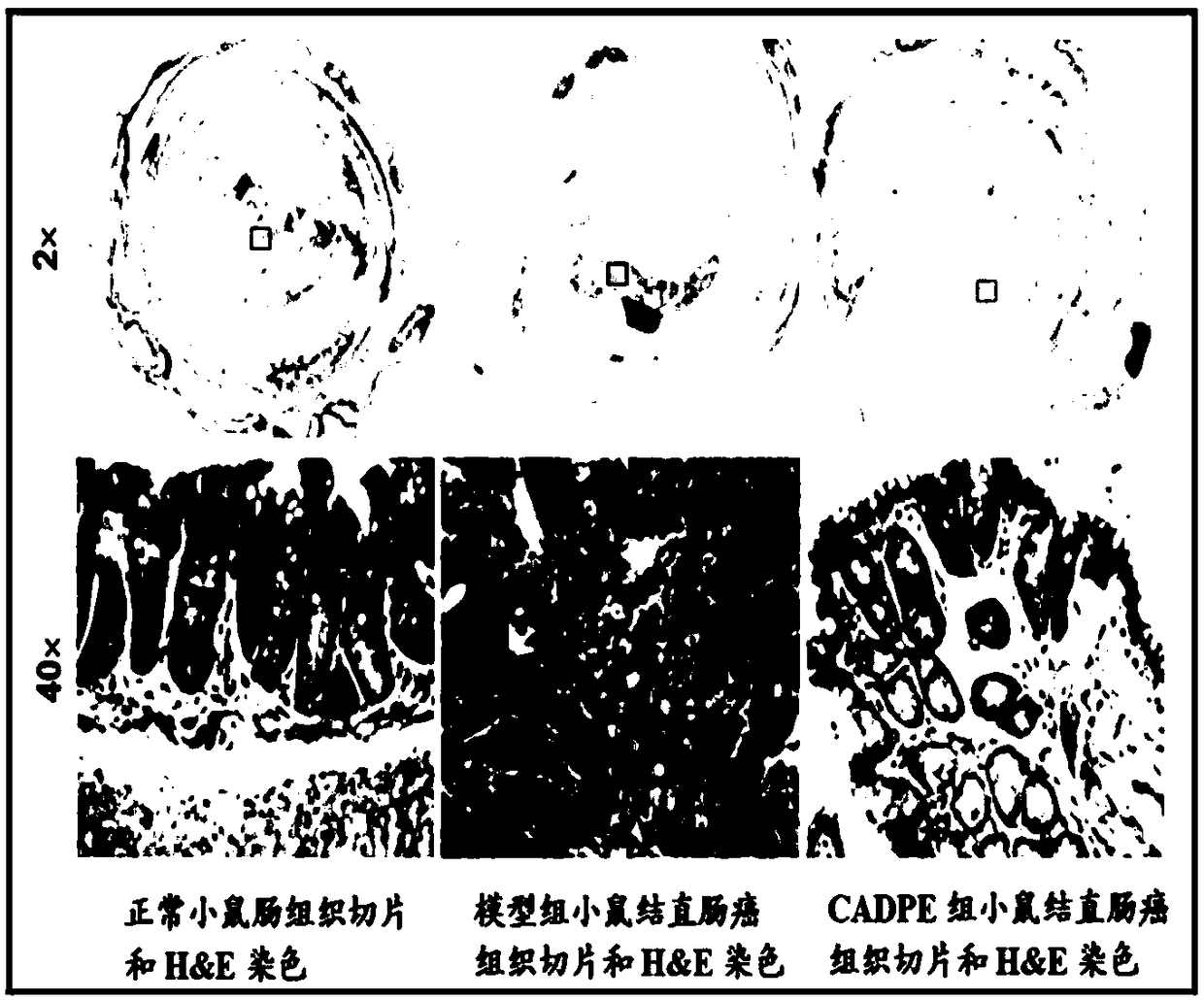
The invention provides application of caffeic acid 3,4-dyhydroxy phenethyl ester (CADPE) to preparing a medicine for preventing colorectal cancer. The medicine is prepared from CADPE as an active component and a pharmaceutically acceptable carrier. The CAPDE is capable of effectively preventing early-stage occurrence of ICR (Institute for Cancer Research) mouse colorectal cancer induced by 1,2-dimethylhydrazine (DMH) and dextran sulphate sodium salt, has no conspicuous toxicity, and has the function mechanisms of inhibiting inflammatory cell invasion and infiltration and inhibiting NF-KappaB expression in monocyte, inhibiting expression of beta-catenin in aberrant crypt, reducing expression of cell proliferin PCNA and Cyclin D1, an anti-apoptoasis protein surviving and a cancer gene c-Myc,and inhibiting tumor vessel proliferation. Novel treatment means are provided for clinical prevention and treatment on colorectal cancer.
Owner:ZHEJIANG UNIV
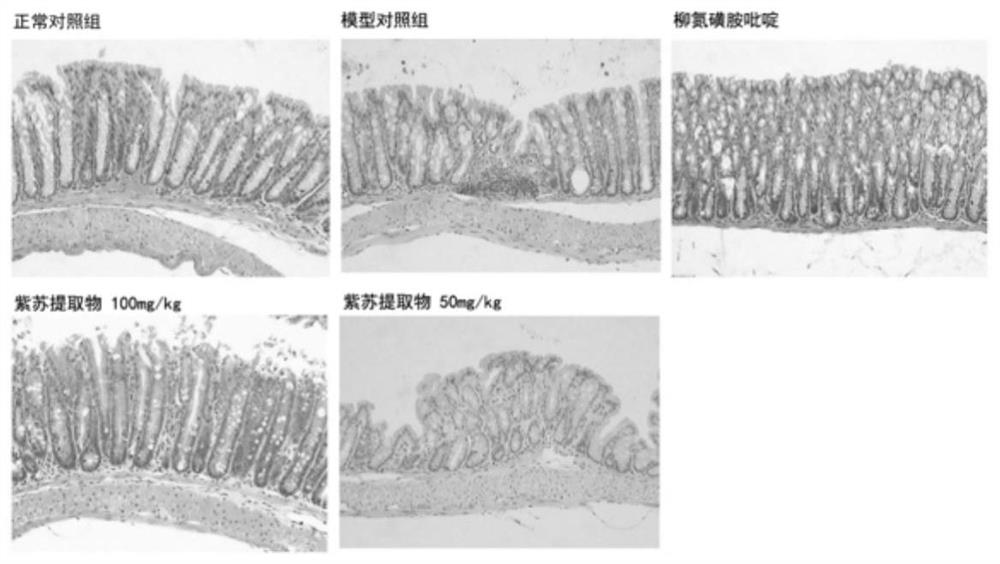
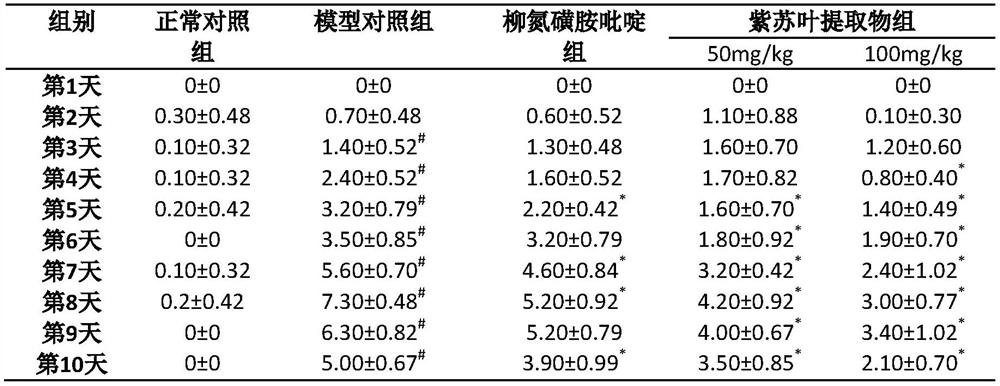
Application of folium perillae extracts in preparation of medicine for treating and/or preventing inflammatory bowel disease
ActiveCN112076249AAll natural ingredientsSmall toxicityAntipyreticAnalgesicsBowels diseasesPharmaceutical drug
The invention discloses application of folium perillae extracts in preparation of a medicine for treating and / or preventing the inflammatory bowel disease. The folium perillae extracts disclosed by the invention have a reverse effect on diarrhea, hematochezia, weight loss and the colon position pathological injuries of a mouse with inflammatory bowel disease induced by low molecular weight dextransulfate, can obviously inhibit secretion of model mouse colon tissue cell factors NO, IFN-gamma, IL-4, IL-10 and IL-17, and can obviously inhibit the intestinal tissue MPO level of the model mouse.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Application of nostoc commune vauch alcohol extract to preparation of medicines for treating inflammatory bowel disease
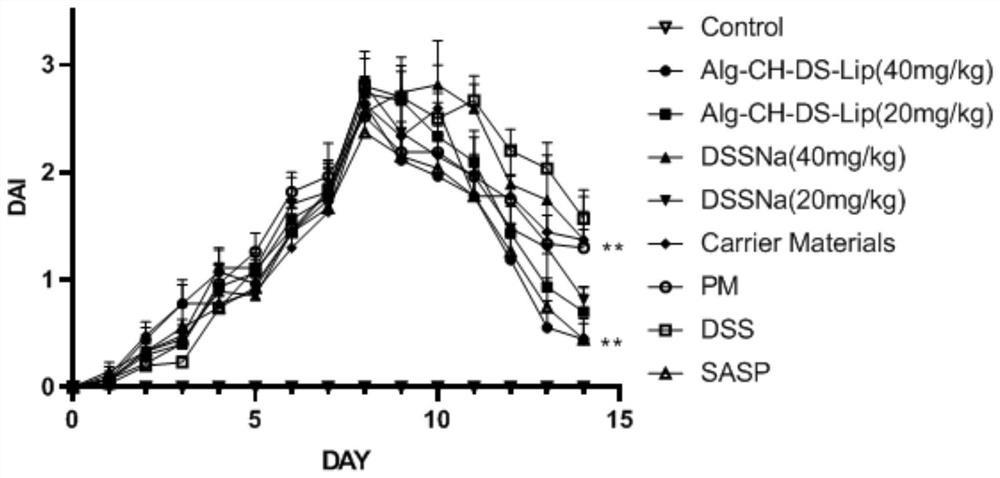
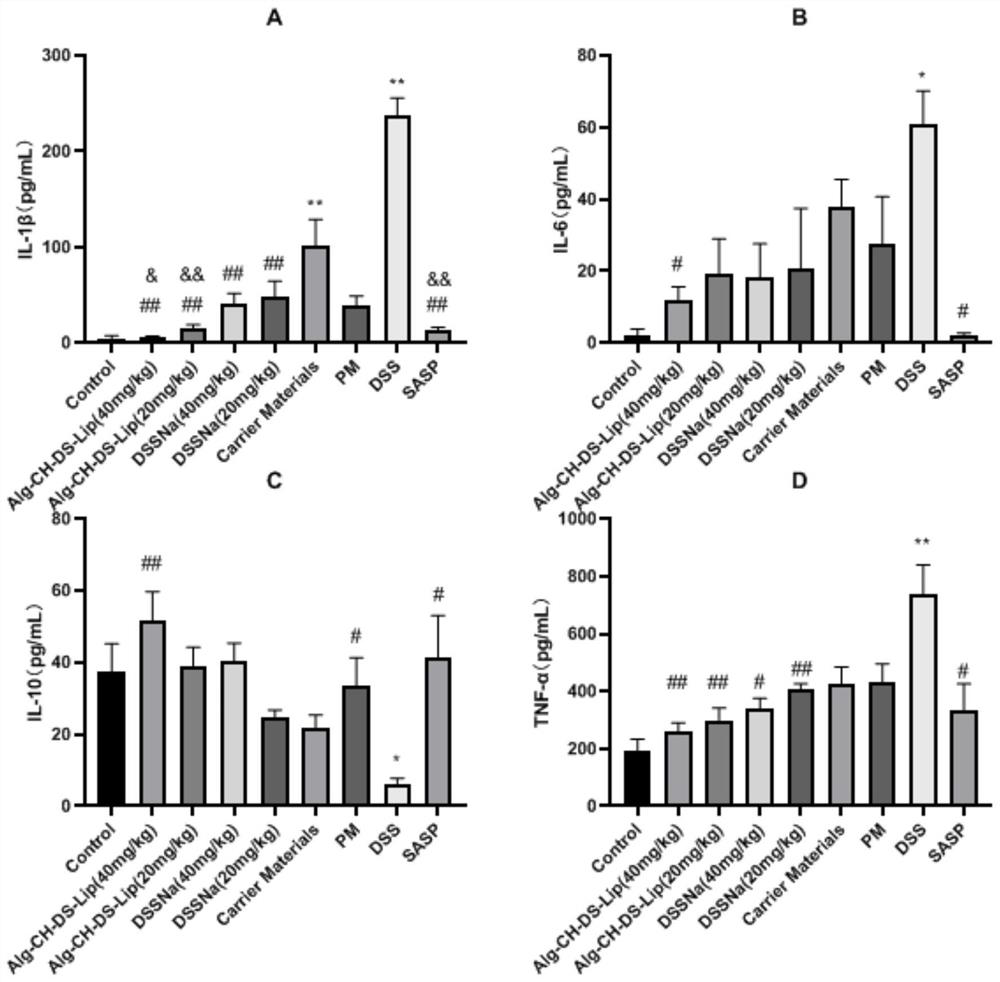
ActiveCN107582574ALose weightImprove severityDigestive systemUnknown materialsInflammatory Bowel DiseasesMouse Colon
The invention discloses application of a nostoc commune vauch alcohol extract to preparation of medicines for treating an inflammatory bowel disease. A preparation method of the nostoc commune vauch alcohol extract comprises the following steps: taking nostoc commune vauch, using low-carbon alcohol as a solvent, extracting under a heating condition, collecting an extract, recycling the solvent toobtain the nostoc commune vauch alcohol extract. Through experiments, the applicant finds that the nostoc commune vauch alcohol extract significantly improves the weight loss of a DSS (dextran sulphate sodium)-induced UC (ulcerative colitis) model mouse, inhibits DAI (disease activity index) score increase, relieves UC-induced mouse colon length reduction and reduces inflammatory cell infiltrationand tissue damage; in addition, the nostoc commune vauch alcohol extract significantly reduces the contents of colon tissue cytokines TNF-alpha and IL-6, and such effect is closely related to activation of a key transcription factor NF-kappaB inhibiting inflammatory signaling pathways. Experimental results of the applicant show that the nostoc commune vauch alcohol extract can be used for treating the inflammatory bowel disease, relieving an inflammatory reaction of the DSS-induced UC model and improving the UC severity.
Owner:GUILIN MEDICAL UNIVERSITY
Phase-change multi-modal nano contrast agent for targeted therapy of vulnerable plaques
PendingCN112656955AReduce phagocytosisProlong blood circulation half-lifeIn-vivo testing preparationsDextranTargeted therapy
The invention discloses a phase-change multi-modal nano contrast agent for targeted therapy of vulnerable plaques. The contrast agent comprises a shell membrane and a core wrapped by the shell membrane, the shell membrane is PLGA-PEG-PLGA, the core is PFH and Fe3O4, the shell membrane is connected with CS (chitosan), and the CS is connected with DS (dextran sulfate). The multi-modal nano contrast agent has a remarkable targeted therapy effect on vulnerable plaques.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
Application of pomegranate rind tannin as well as punicalagin and granatin serving as effective components of pomegranate rind tannin in preparation of medicine for treating ulcerative colitis
PendingCN114272276AHas therapeutic effectGood curative effectOrganic active ingredientsDigestive systemGoblet cellPharmaceutical drug
The invention belongs to the technical field of medicines, and discloses pomegranate rind tannin and application of punicalagin and granatin serving as effective components of the pomegranate rind tannin in preparation of medicines for treating ulcerative colitis. After the pomegranate rind tannin is used for treating mouse ulcerative colitis induced by dextran sulfate sodium salt, the body weight, the disease activity index, the colorectum length, the colorectum mucous membrane form and the goblet cell number are improved to a certain extent compared with a model group; therefore, the pomegranate rind tannin has a certain treatment effect on ulcerative colitis. The effective components punicalagin and granatin in the pomegranate rind tannin are independently or jointly administered, so that the pomegranate rind tannin has a very good effect on ulcerative colitis, and drug combination is optimal.
Owner:GUANGDONG UNIV OF TECH
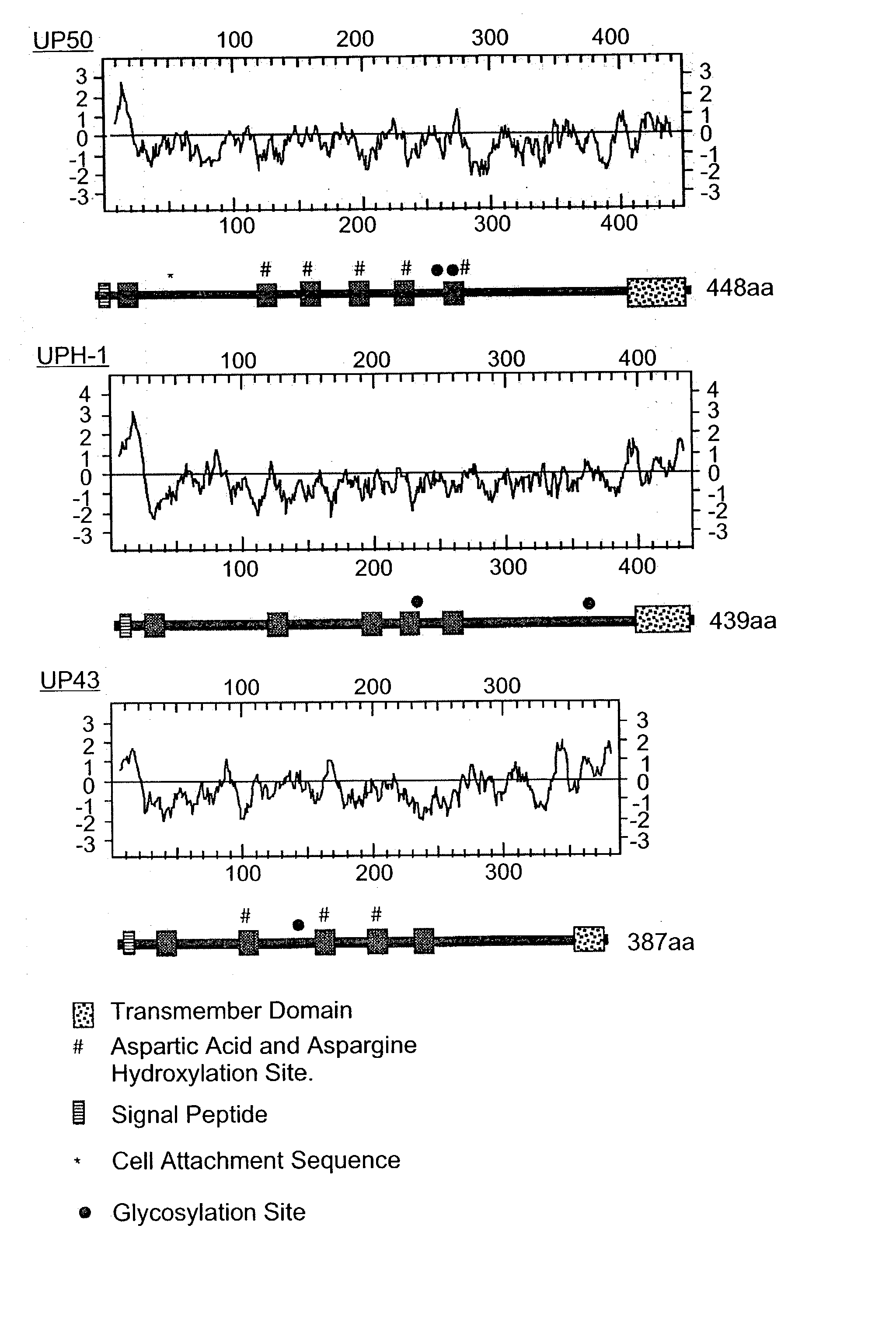
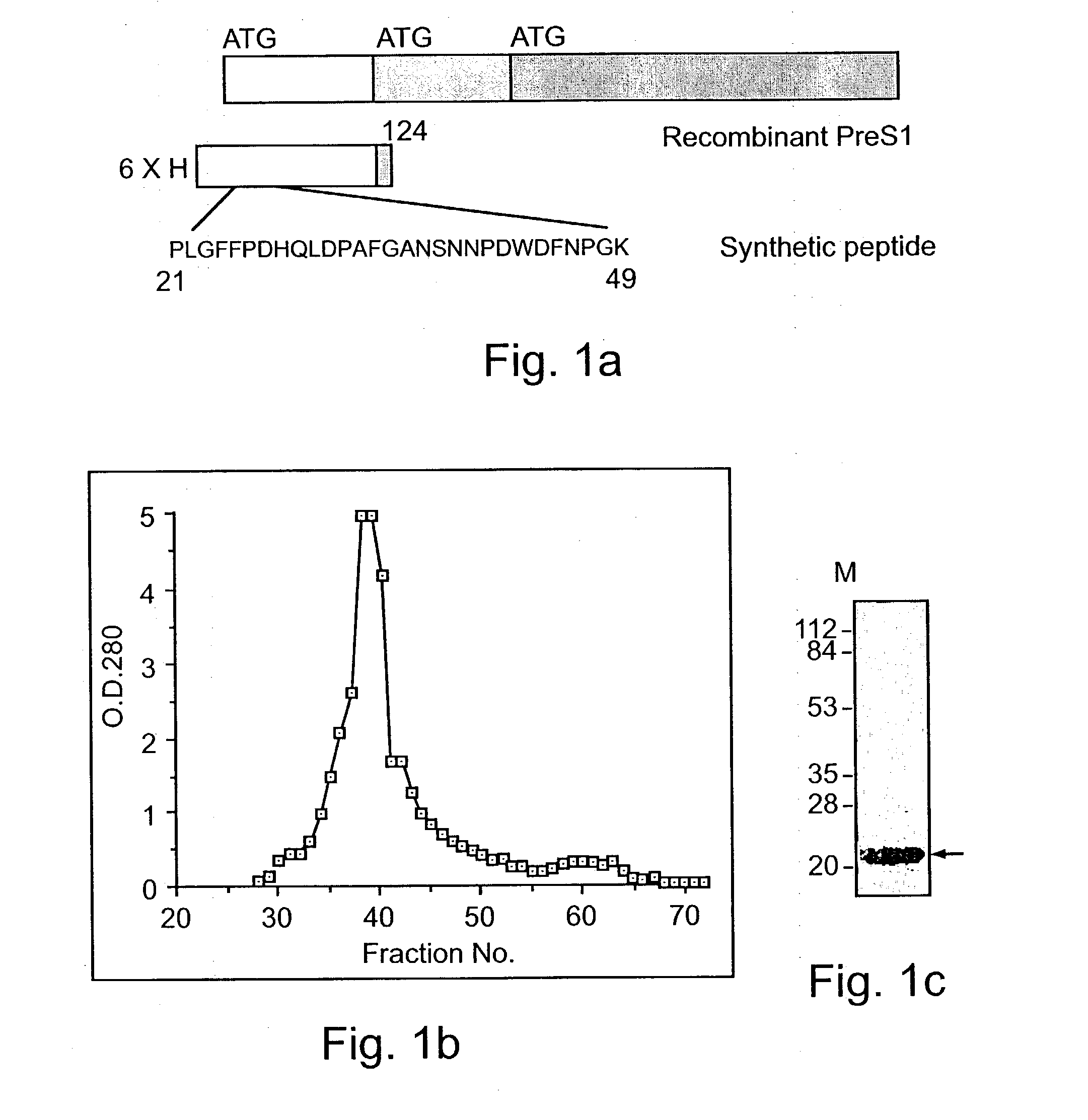
Hepatitis B virus binding proteins and uses thereof
An isolated nucleic acid, a recombinant protein encoded thereby and uses thereof. The isolated nucleic acid including (a) a polynucleotide at least 60% identical to SEQ ID NOs:1, 3, 5 or portions thereof as determined using the Bestfit procedure of the DNA sequence analysis software package developed by the Genetic Computer Group (GCG) at the university of Wisconsin (gap creation penalty-50, gap extension penalty-3); (b) a polynucleotide encoding a polypeptide being at least 60% homologous with SEQ ID NOs:2, 4, 6 or portions thereof as determined using the Bestfit procedure of the DNA sequence analysis software package developed by the Genetic Computer Group (GCG) at the university of Wisconsin (gap creation penalty-50, gap extension penalty-3); or (c) a polynucleotide hybridizable with SEQ ID NOs:1, 3, 5 or portions thereof at 68° C. in 6xSSC, 1% SDS, 5x Denharts, 10% dextran sulfate, 100 mug / ml salmon sperm DNA, and <32>p labeled probe and wash at 68° C. with 3xSSC and 0.1% SDS.
Owner:YEDA RES & DEV CO LTD
Application of dalbergia pinnata essential oil to preparation of medicine for treating or preventing ulcerative colitis
ActiveCN108785362ASymptoms improvedPrevent weight lossDigestive systemPlant ingredientsUlcerative colitisDalbergia pinnata
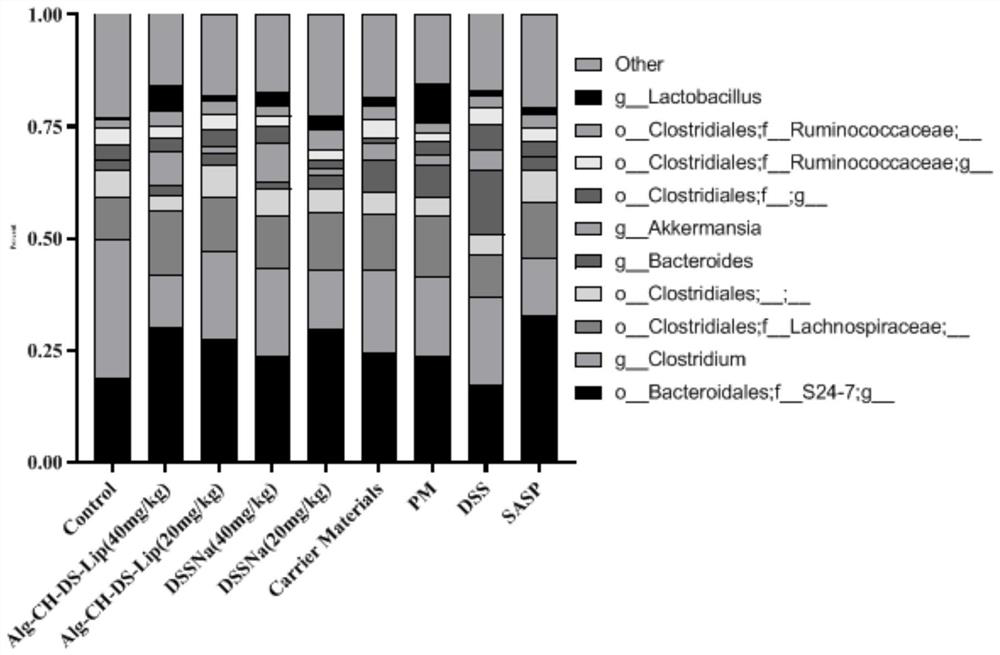
The invention provides application of dalbergia pinnatad essential oil to preparation of a medicine for treating or preventing ulcerative colitis. The essential oil can effectively inhibit DSS (dextran sulphate sodium) from inducing loss of weight of mice suffering from ulcerative colitis, reduce the disease activity index of the mice suffering from DSS induced ulcerative colitis, improve the overall score of the colon tissue and has the effect more excellent than that of the existing clinical medicine SASP (salazosulfapyridine) for ulcerative colitis.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
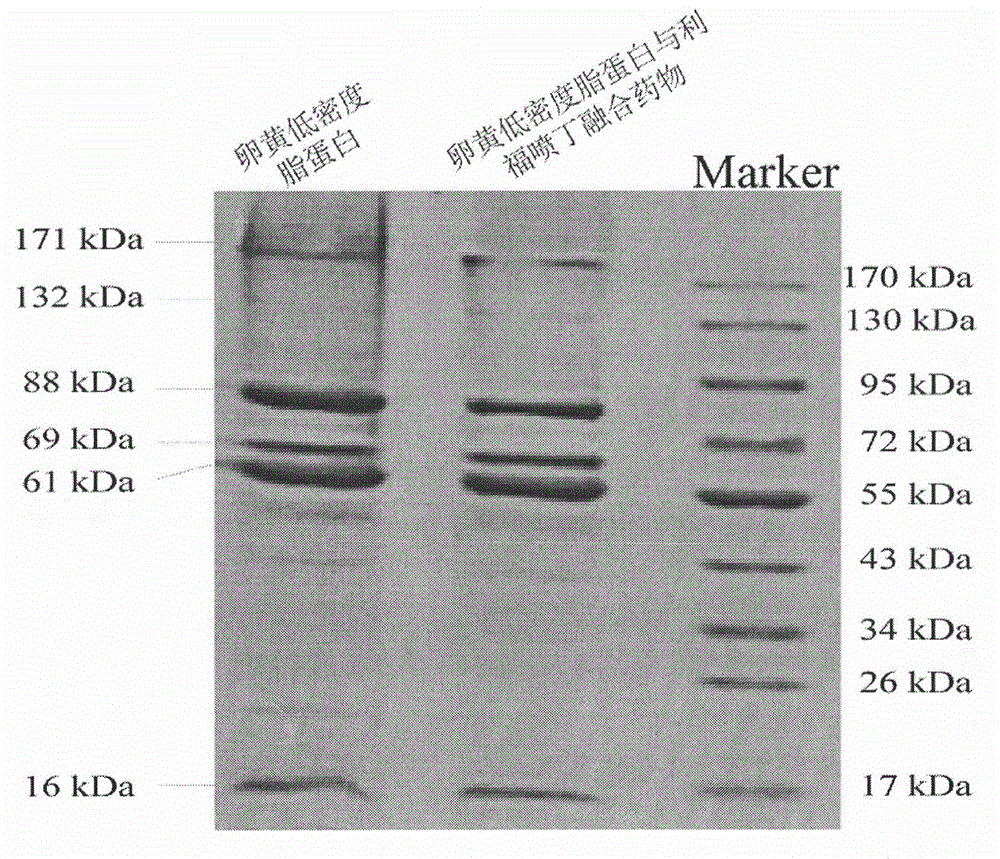
Extraction of yolk low density lipoprotein and fusion technology for yolk low density lipoprotein and Rifapentine
The invention relates to a novel extraction method for yolk low density lipoprotein and a fusion technology for the yolk low density lipoprotein and Rifapentine. The novel extraction method for the yolk low density lipoprotein comprises the following steps: (1) preparation of yolk slurry; (2) extraction of the yolk low density lipoprotein through dextran sulfate cellulose affinity chromatography; and (3) dialysis for removal of salt. The fusion technology for yolk low density lipoprotein and Rifapentine comprises the following steps: (1) preparation of an aqueous Rifapentine solution; (2) fusion of the yolk low density lipoprotein and Rifapentine; (3) purification of a fusion drug through dextran sulfate cellulose affinity chromatography; and (4) dialysis for removal of salt. The extraction method is simple, has mild reaction conditions and high extraction efficiency and uses simple and easily controllable equipment; the obtained yolk low density lipoprotein and the fusion drug of the yolk low density lipoprotein and Rifapentine have good stability; the extraction method is applicable to extraction of the yolk low density lipoprotein; and the yolk low density lipoprotein is applicable to fields like drug delivery, processing of food emulsifier, etc.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
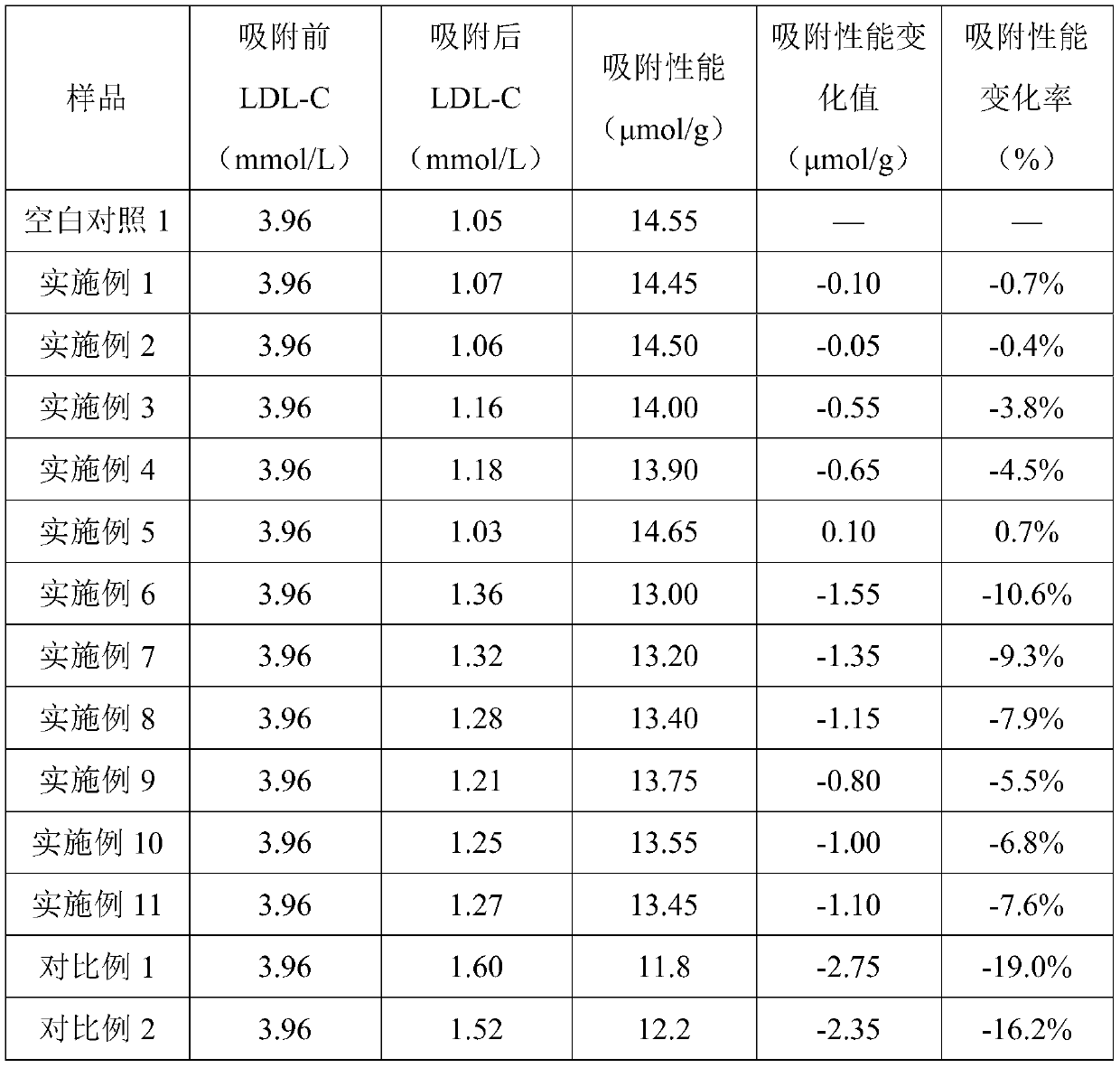
Filling liquid for stabilizing adsorption performance of adsorbent and application thereof
ActiveCN110665478ADecrease rate of adsorption performance is reducedImprove adsorption capacityIon-exchange process apparatusOther chemical processesA lipoproteinSorbent
The invention discloses a filling liquid for stabilizing adsorption performance of an adsorbent and application thereof. The filling liquid comprises a stabilizer and a base liquid, wherein the stabilizer is one or more of dextran and derivatives thereof, and the mass volume ratio of the stabilizer to the base liquid is 0.02-2.5g: 100 ml. The filling liquid provided by the invention can obviouslyreduce the reduction rate of the adsorption performance of the adsorbent after high-pressure steam sterilization, and is suitable for various adsorbents, especially low density lipoprotein adsorbentswith dextran sulfate as a ligand.
Owner:WUHAN RUIFA MEDICAL DEVICES CO LTD
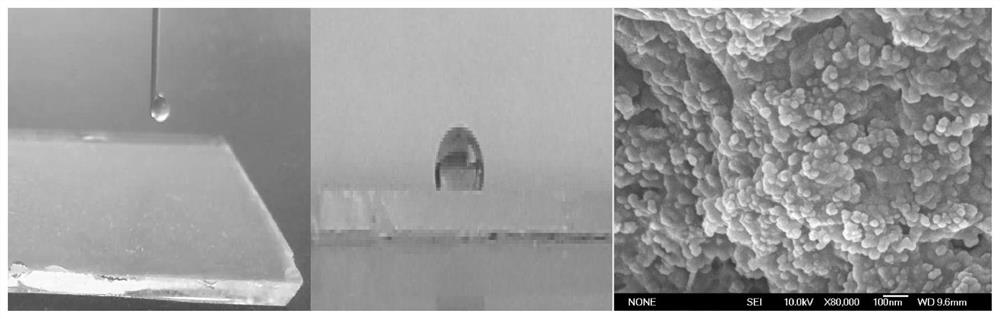
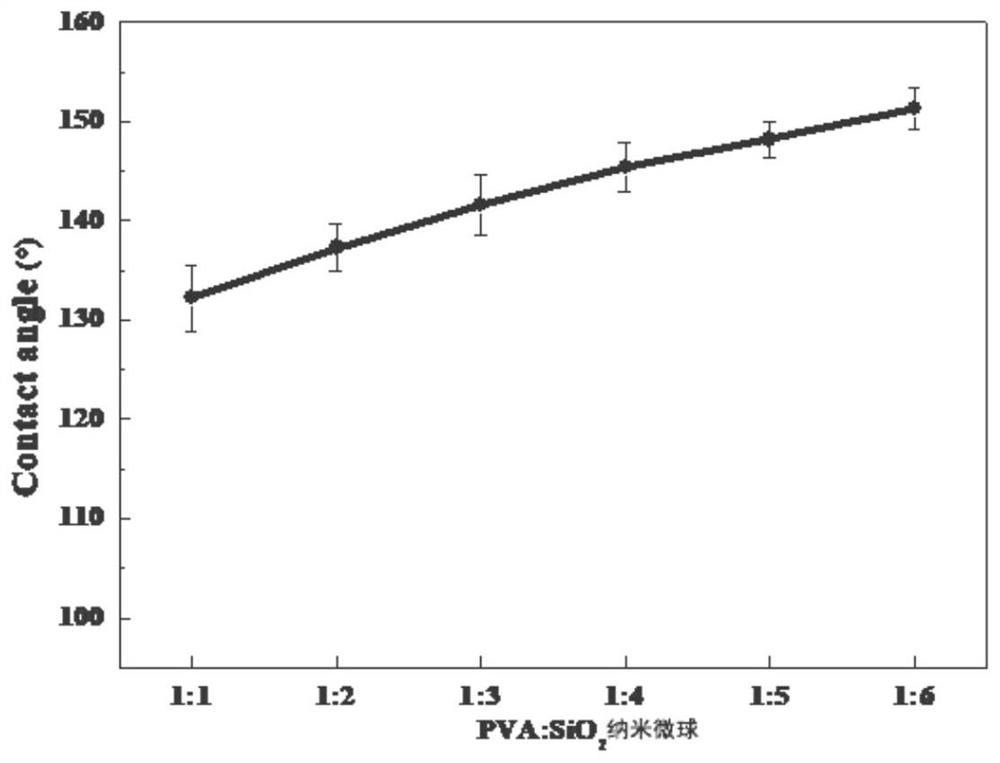
Fluorinated PVA/SiO2 super-hydrophobic membrane and preparation method thereof
The invention provides a fluorinated PVA / SiO2 super-hydrophobic membrane and a preparation method thereof. The preparation method comprises the following steps of: adding a proper amount of SiO2 intoa chitosan acid solution, and carrying out ultrasonic treatment to prepare a chitosan / SiO2 solution; dropwise adding a dextran sulfate alkali solution into the chitosan / SiO2 solution serving as a basesolution at a certain titration rate, and carrying out stirring, standing and centrifuging to freeze-dry SiO2-containing nano microspheres on the lower layer; preparing a PVA solution; and mixing theSiO2-containing nano microspheres into the PVA solution, uniformly leveling the mixed solution on a glass sheet to obtain a PVA / chitosan / SiO2 membrane, and putting the PVA / chitosan / SiO2 membrane intoan FAS ethanol solution for fluorination to obtain the fluorinated PVA / SiO2 super-hydrophobic membrane. The invention also provides a fluorinated PVA / SiO2 super-hydrophobic membrane. According to thefluorinated PVA / SiO2 super-hydrophobic membrane provided by the invention, the technical problem of poor hydrophobicity of a membrane prepared from SiO2 and PVA conventionally is solved.
Owner:TAIYUAN UNIV OF TECH
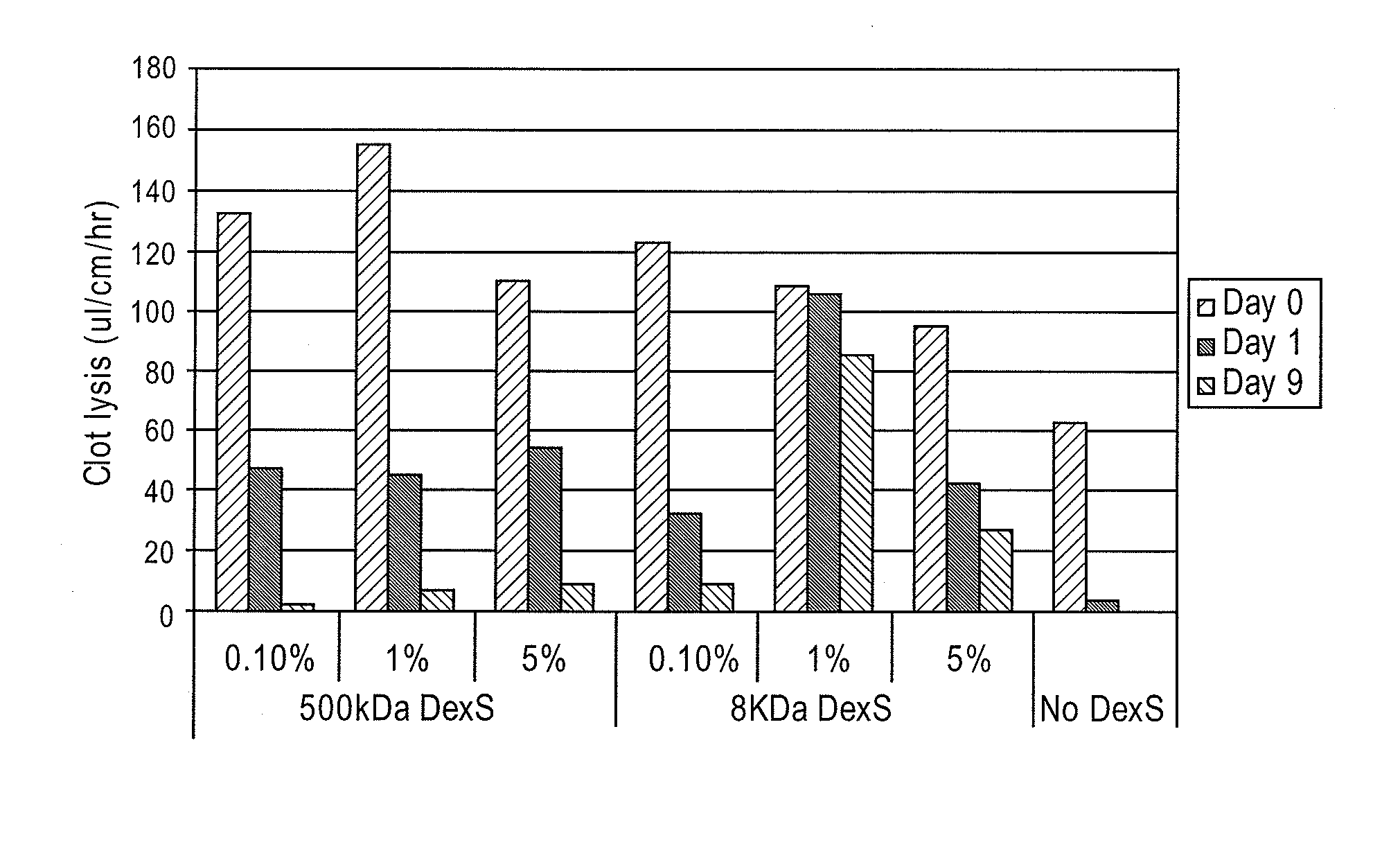
Stabilized enzyme compositions
ActiveUS8545459B2Reduce formationReduce microbial growthAntibacterial agentsPeptide/protein ingredientsMedical deviceMedical treatment
A medical device includes a base material having an immobilized fibrinolytic enzyme and dextran sulfate. The dextran sulfate has a molecular weight that is less than 40 kilo dalton (kDa). The medical device is formed from at least a base material. A fibrinolytic enzyme is immobilized on the base material. The fibrinolytic enzyme is stabilized with a dextran sulfate having a molecular weight of less than 40 (kDa).
Owner:TELEFLEX MEDICAL INC +1
Sample pretreatment solution for quantitative vitamin D detection and use method of solution
The invention discloses a sample pretreatment solution for quantitative vitamin D detection. The solution comprises normal saline, dextran sulfate sodium salt and a beta-isotropism blocking agent. Thedextran sulfate sodium salt and the beta-isotropism blocking agent can be used to fully separate 25-hydroxyvitamin D to be analyzed from binding protein to a certain extent. Non-specific interferenceeffect in a serum matrix is reduced, the sample can be well separated in the further separation and purification process, a high-quality to-be-analyzed object can be obtained from the treated sample,and the quantitative detection accuracy of the vitamin D is improved.
Owner:重庆黄嘉同怡科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com