Binding of pathological forms of prion proteins
A protein and form technology, applied in disease diagnosis, peptide preparation method, peptide source, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Application of biotinylated pentosan polysulfate and subsequent affinity capture from inferior Normal Prion
[0071] introduce
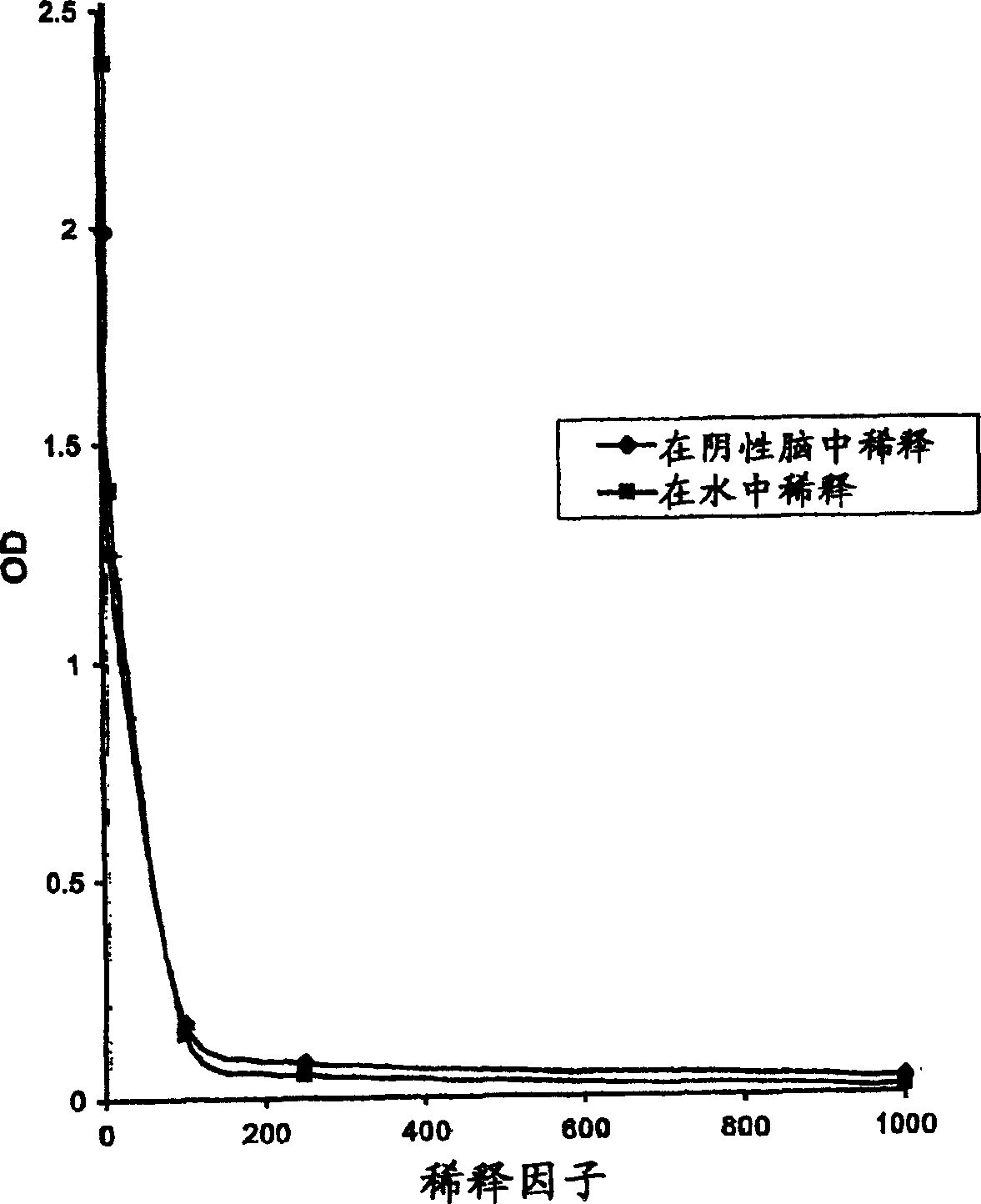
[0072] Biotin was conjugated to pentosan polysulfate using standard chemistry. This biotinylated pentosan polysulfate was bound to the inferior prion protein in the brain homogenate, after binding, the pentosan polysulfate / prion was captured using streptavidin-derivatized superparamagnetic beads Complex. The captured inferior prions were then eluted from these beads and treated with an immune-based Bio-RadPlatelia TM Measured with BSE detection kit; this kit cannot distinguish between normal and inferior prion protein, and can give signals of both proteins. A study was carried out on a set of two BSE-infected and two non-infected bovine brains to illustrate that under the specific conditions described, pentosan polysulfate-specific capture of prion proteins in brain homogenates can be specific .
[0073] method
[007...
Embodiment 2
[0101] Embodiment 2: Use immobilized biotinylated pentosan polysulfate to separate normal prion and bad prion protein
[0102] introduce
[0103] Biotin was conjugated to pentosan polysulfate using standard chemistry. Streptavidin-derivatized superparamagnetic beads were coated with this biotinylated pentosan polysulfate. The coated beads were then used to specifically capture the prion protein in the brain homogenate. Subsequently, the captured inferior prion protein was eluted from these beads and immuno-based Bio-Rad Platelia TM It is detected by the BSE detection kit; this kit cannot distinguish between normal and inferior prion proteins, and can give signals of both proteins. A group of 3 BSE-infected and 3 non-infected bovine brains was studied and used to demonstrate that under the special conditions described, pentosan polysulfate can specifically capture the prion protein in these brain homogenates.
[0104] method
[0105] Preparation of magnetic beads ...
Embodiment 3
[0133] Example 3. Biotinylation of PPS
[0134] Rationale for this method
[0135] Substitution of approximately one-tenth of the sugar residues in the xylan backbone of pentosan sulfate with uronic acid residues, followed by substitution of methyl esters at some of the carboxyl groups, thus present in this molecule A large number of free carboxyl groups can be derivatized with carbodiimide to form active esters. These can then be substituted with amino groups to create an amide bond. In this particular case, EDC and NHS were chosen to form the active ester and biotin hydrazide was chosen as the amino group. Two reactions were performed, in the first reaction, biotin hydrazide was initially present without any NHS addition, and in the second reaction, NHS / EDC was reacted with PS and biotin hydrazide simultaneously.
[0136] Material
[0137] Pentosan sulfate (Norton Healthcare) was a gift from Stephen Dealer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com