Method for preparing silica gel supported dithiocarbamate heavy metal chelating resin
A technology of dithioamino and chelating resins, which is applied in chemical instruments and methods, and other chemical processes, can solve the problems of low adsorption capacity of chelating resins and low number of dithiocarbamate functional groups, and achieve improved Large adsorption capacity, large adsorption capacity, and good mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
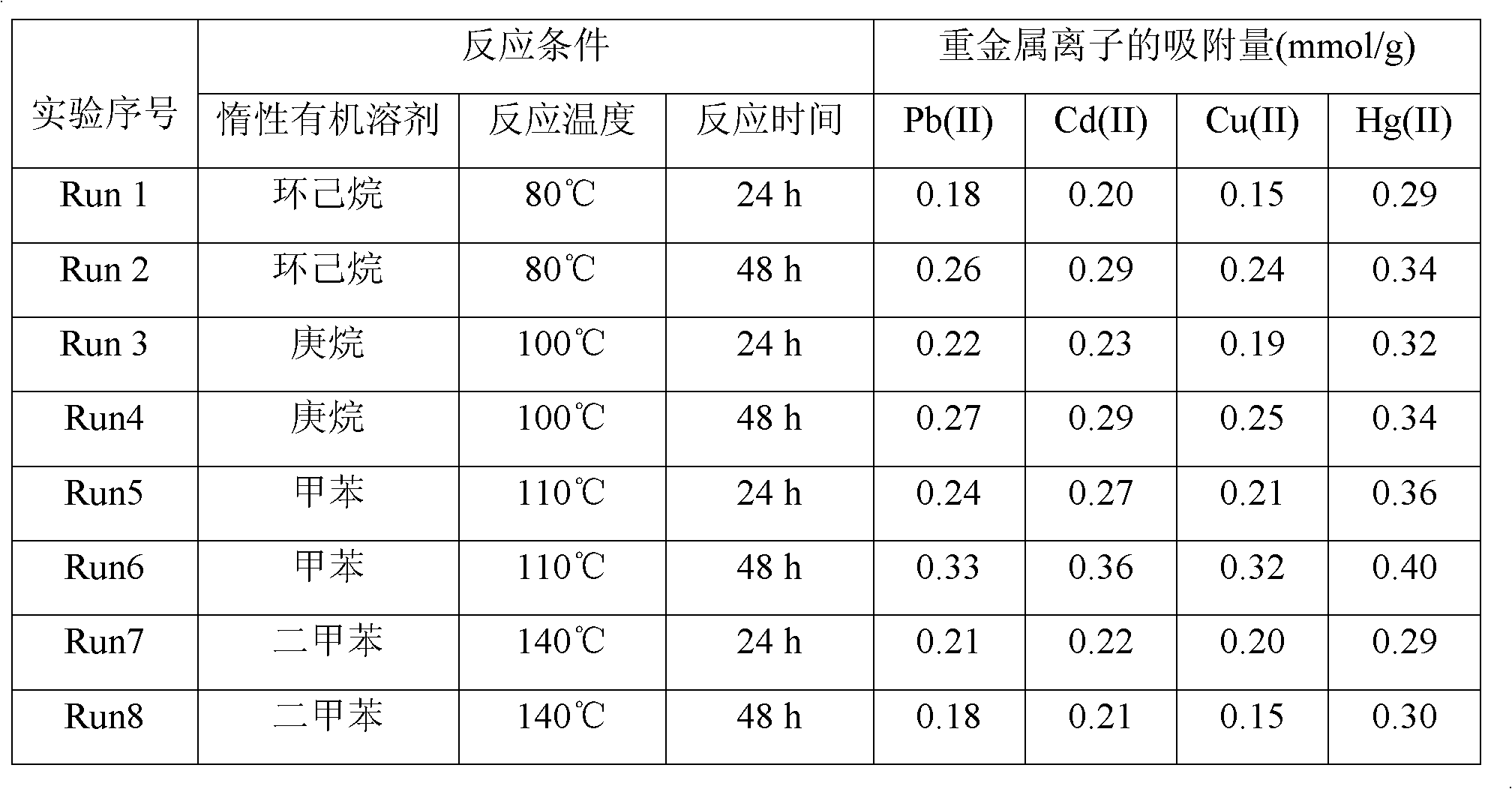
[0020] Embodiment 1 adopts different inert organic solvents to prepare silica gel-loaded dithiocarbamate chelating resin
[0021] (1) Take 30g of chromatographic silica gel in a 500ml three-necked bottle, add 1mol / L HNO 3 The solution was 200ml, mechanically stirred at 50°C for 6h, and after the reaction, the solid product was obtained by suction filtration, which was washed with deionized water and methanol until neutral, and dried at room temperature for 12h to obtain activated silica gel.
[0022] (2) Take 20g of the activated silica gel obtained in step (1), 150ml of cyclohexane, heptane, toluene or xylene and add it to a 500ml three-necked flask, stir mechanically, and heat the reaction system in an oil bath to 80-140°C; 80ml Mix cyclohexane, heptane, toluene or xylene with 20ml of γ-chloropropyltriethylsilane evenly and drop them into the three-neck bottle drop by drop. During the whole reaction process, keep the system temperature at 80-140°C and reflux under nitrogen a...
Embodiment 2
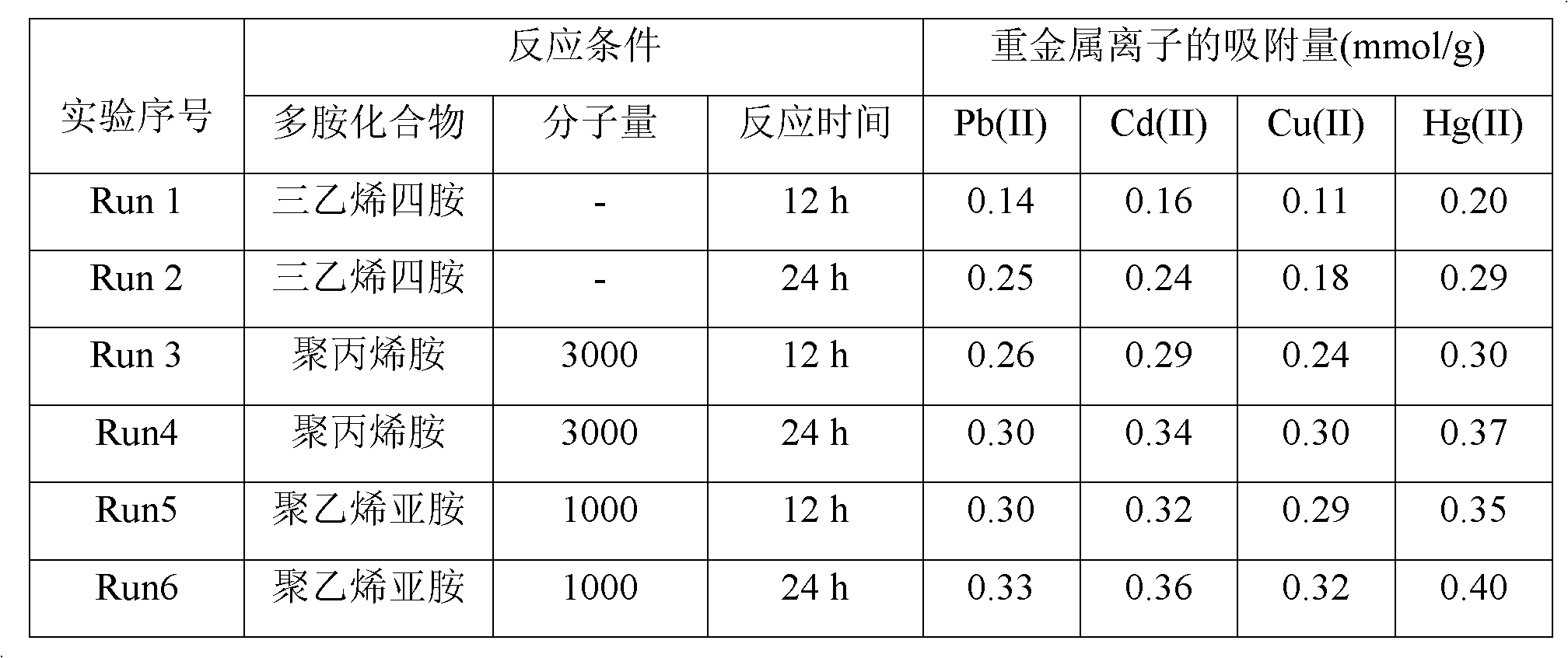
[0027] Embodiment 2 adopts different polyamine compounds to prepare silica gel-loaded dithiocarbamate chelating resin
[0028] (1) Take 30g of chromatographic silica gel in a 500ml three-necked bottle, add 1mol / L HNO 3 The solution was 200ml, mechanically stirred at 50°C for 6h, and after the reaction, the solid product was obtained by suction filtration, which was washed with deionized water and methanol until neutral, and dried at room temperature for 12h to obtain activated silica gel.
[0029] (2) Take 20g of the activated silica gel obtained in step (1) and 150ml of toluene into a 500ml three-necked flask, mechanically stir, and heat in an oil bath to raise the temperature of the reaction system to 110°C; mix 80ml of toluene with 20ml of γ-chloropropyltriethylsilane After uniformity, it was dropped into a three-necked bottle drop by drop. During the whole reaction process, the temperature of the system was kept at 110° C., and the nitrogen atmosphere was refluxed for 48 h...
Embodiment 3
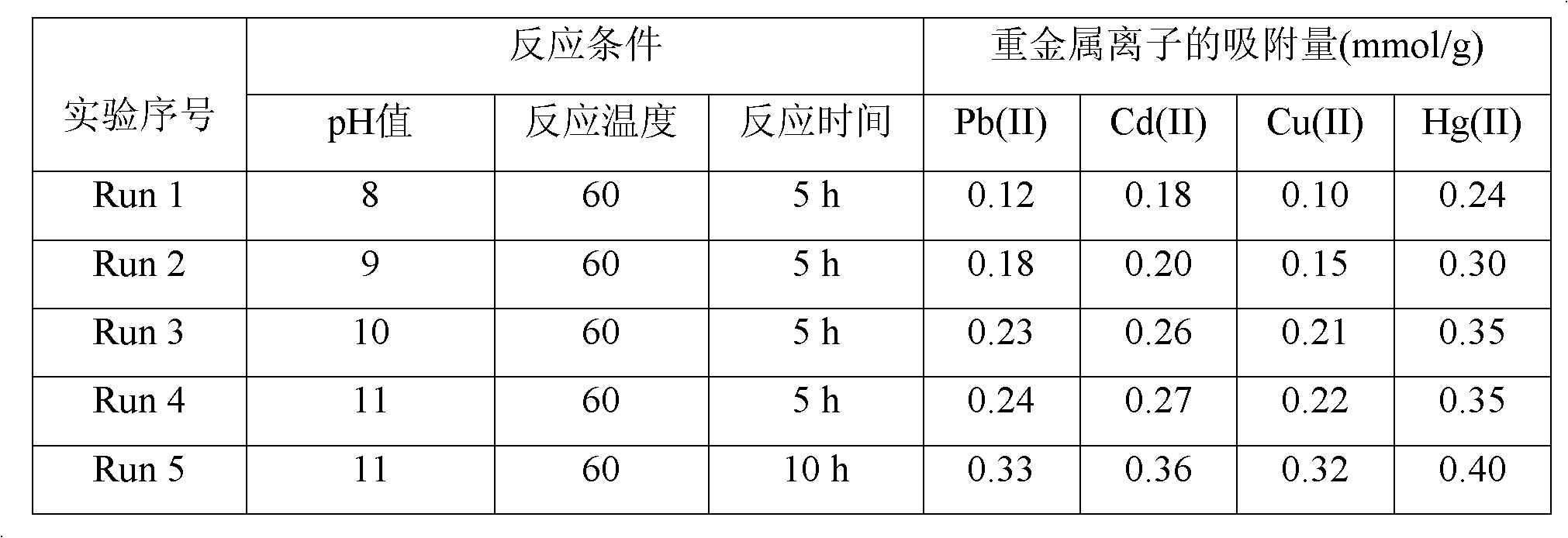
[0034] Example 3 Controlling the different pH values of the reaction system to prepare silica gel-loaded dithiocarbamate chelating resins
[0035] (1) Take 30g of chromatographic silica gel in a 500ml three-necked bottle, add 1mol / L HNO 3 The solution was 200ml, mechanically stirred at 50°C for 6h, and after the reaction, the solid product was obtained by suction filtration, which was washed with deionized water and methanol until neutral, and dried at room temperature for 12h to obtain activated silica gel.
[0036] (2) Take 20g of the activated silica gel obtained in step (1) and 150ml of toluene into a 500ml three-necked flask, mechanically stir, and heat in an oil bath to raise the temperature of the reaction system to 110°C; mix 80ml of toluene with 20ml of γ-chloropropyltriethylsilane After uniformity, it was dropped into a three-necked bottle drop by drop. During the whole reaction process, the temperature of the system was kept at 110° C., and the nitrogen atmosphere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com