Recombinant human lactoferrin (rhLF) silkworm chrysalis powder as well as preparation method and application thereof
A technology of silkworm chrysalis powder and protein, which is applied in the field of biomedicine, can solve the problems of restricting drug efficacy, inconvenient application of dosage forms, and low bioavailability, and achieve good medicinal value, high application value, and good biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
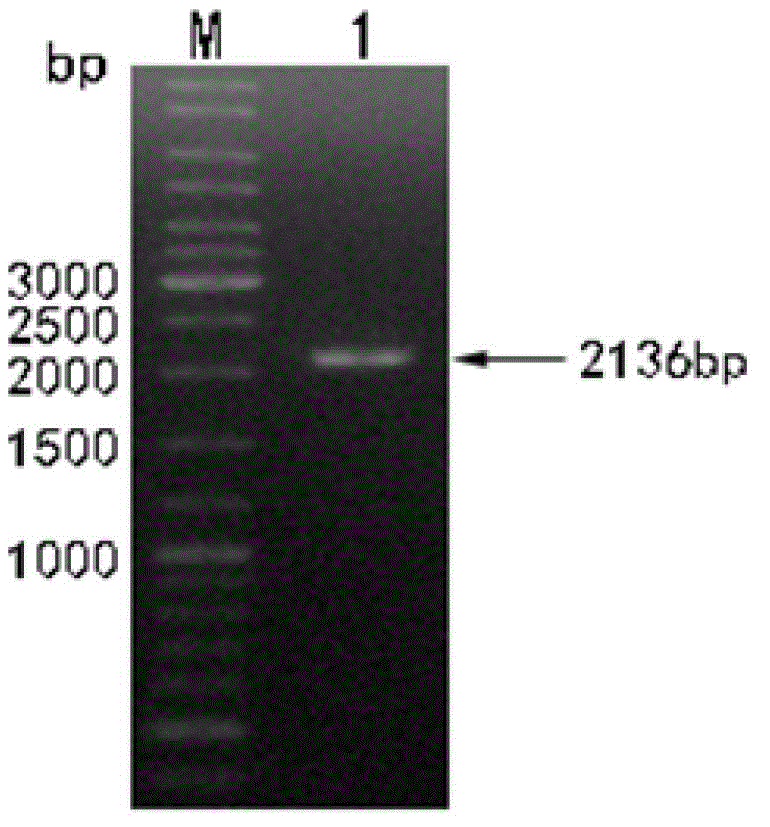
[0042] Embodiment 1: Obtaining of hLF gene (its nucleotide sequence is shown in SEQ ID NO:1)
[0043]Design a pair of primers according to the gene sequence of hLF (gene accession number: AY875691.1) provided on NCBI:
[0044] hLF F: 5'-GCGCTCGAGATGAAACTTGTCTTCC-3'
[0045] hLF R: 5'-CGCGGTACCTTACTTCCTGAGGAATTC-3'
[0046] Recombinant Functional Human Lactoferrin Expressed in Baculovirus System(J). Acta Biochimica et Biophysica Sinica, 2006, 38 (3): 201– 206) as a template, with hLF F and hLF R as upstream and downstream primers, PCR amplification is carried out, and the specific reaction system and reaction procedure are as follows:
[0047] PCR reaction system:
[0048] 10×Taq buffer 5 μL
[0049] 10 mM dNTPs 1 μL
[0050] hLFF 2.5 μL
[0051] hLFR 2.5 μL
[0052] Template 1 μL
[0053] Taq enzyme 1 μL
[0054] wxya 2 O 37 μL
[0055] Reaction procedure:
[0056]
[0057] After the reaction was completed, the amplified fragment was identified by electrophoresi...
Embodiment 2
[0058] Example 2: Construction of recombinant transfer plasmid pFastBac1-hLF
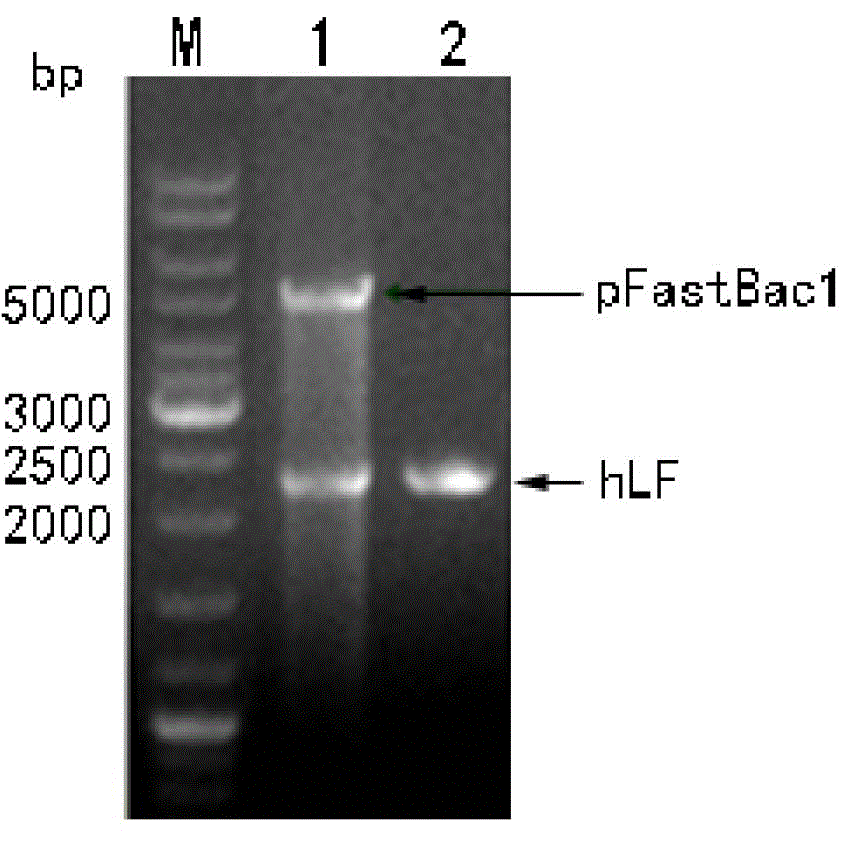
[0059] The pFastBac 1 vector (purchased from Invitrogen) was used xho I and KpnI (purchased from Fermentas Company) After double enzyme digestion, the large fragment was recovered and ligated with the purified hLF PCR product to construct the recombinant transfer plasmid pFstBac1-hLF. The correct gene sequence was identified by restriction analysis and bidirectional sequencing, indicating that the recombinant transfer plasmid was successfully constructed. figure 2 It is the electrophoresis figure of the double enzyme digestion and PCR identification of the recombinant transfer plasmid pFastBac1-hLF.
Embodiment 3
[0060] Embodiment 3: Obtaining of Bombyx mori recombinant baculovirus Bm-rhLF
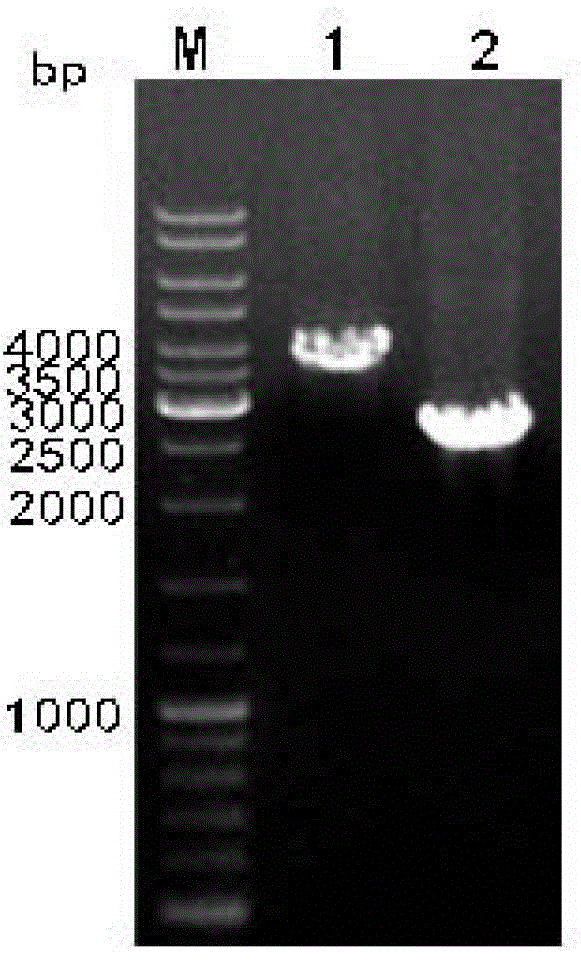
[0061] The recombinant transfer plasmid pFastBac1-hLF, which was identified as a successful recombination, was transformed into Escherichia coli DH10Bac competent cells containing the baculovirus shuttle vector Bacmid (purchased from Invitrogen Company), in the presence of kanamycin, gentamicin, tetracycline, X-gal and IPTG was cultured on an LB culture plate (purchased from Shanghai Sangon Biotechnology Co., Ltd., operated according to the instructions), and homologous recombination was carried out by transposition (the hLF sequence on pFastBac 1-hLF was inserted into the multi-cloning site of Bacmid by homologous transposition After 48 hours of culture in the dark, the white spot was picked, and the white spot was continued to be shaken in the LB culture medium containing tetracycline, kanamycin, gentamycin, X-gal and IPTG for 48 hours, and then treated with isocyanide The recombinant baculovirus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com