Application of pomegranate rind tannin as well as punicalagin and granatin serving as effective components of pomegranate rind tannin in preparation of medicine for treating ulcerative colitis
A technology for ulcerative colitis and pomegranate peel tannin, applied in the field of medicine, can solve problems such as deficiency, and achieve the effect of good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1: Prepare pomegranate peel tannin (PPT)
[0087] 1) Weigh 200g of dried pomegranate peel, crush it, add 5000mL of 60% ethanol water solution by volume, soak in 60% ethanol water solution for more than 2 hours before extraction, improve extraction efficiency, ultrasonically assisted extraction for 50min, repeat Extract three times, collect the filtrate by suction filtration after each extraction, and combine the filtrate obtained three times, which is the crude extract of pomegranate peel;
[0088] 2) Concentrate the crude extract of pomegranate peel to 300mL solution by rotary evaporation until there is no obvious smell of ethanol;
[0089] 3) Redissolve the concentrated pomegranate peel extract in an equal volume of water, slowly add a 3% gelatin aqueous solution by volume percentage until no white flocculent precipitates are produced, and filter with suction to obtain a white precipitate, which is the formation of tannin and gelatin the compound;
[0090...
Embodiment 2
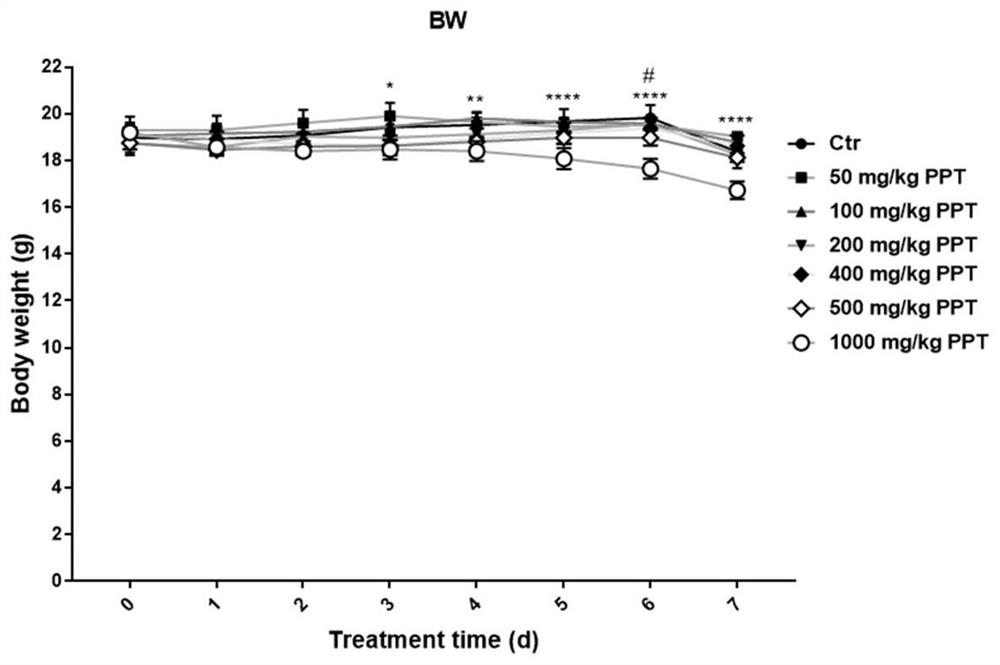
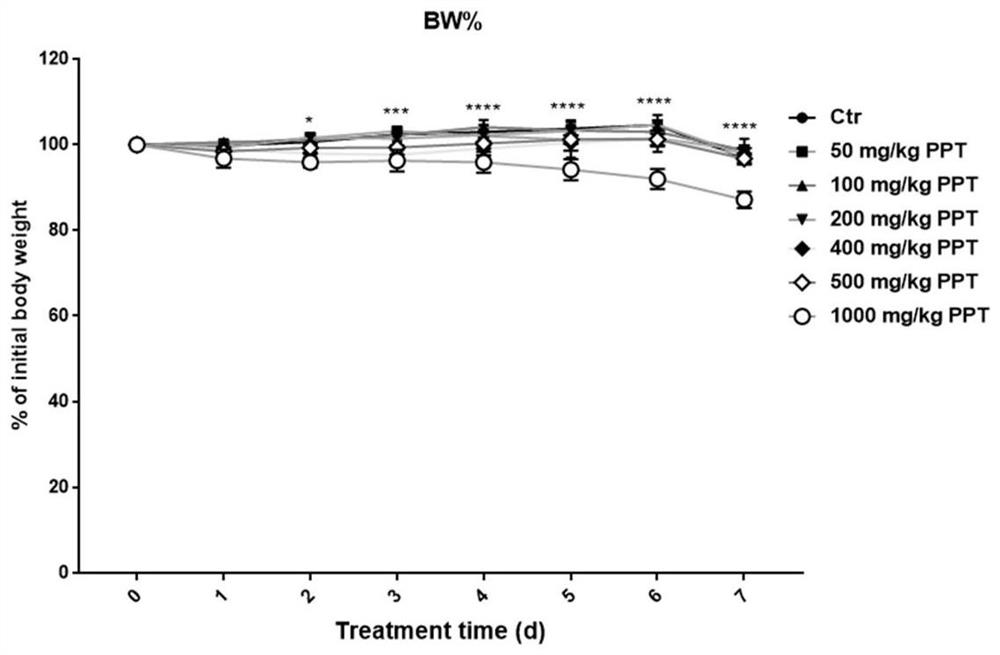
[0094] Embodiment 2: the determination of pomegranate peel tannin dosage
[0095] 1) The pomegranate peel tannins prepared in Example 1 were prepared into dilutions of 5, 10, 20, 40, 50, and 100 mg / mL with PBS buffer solution, respectively.
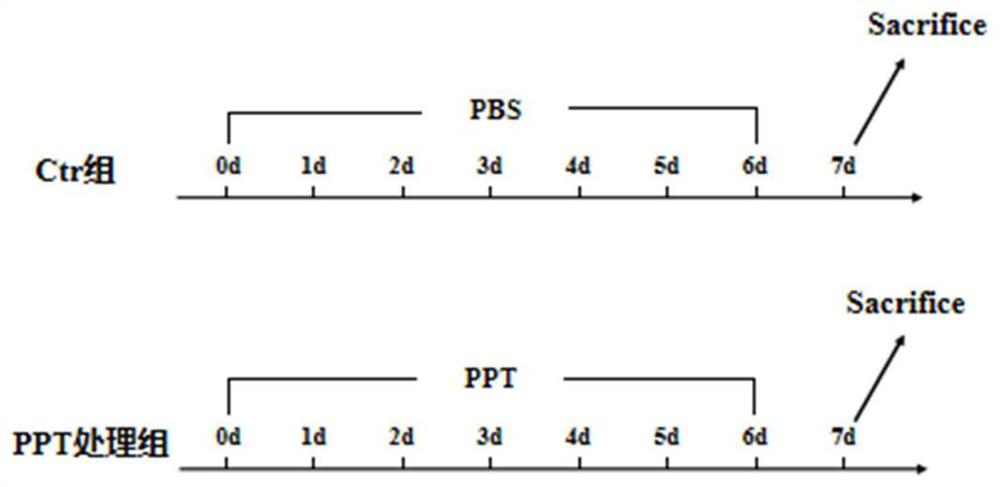
[0096] 2) C57 BL / 6 male mice aged 6 weeks and weighing 14-16 g were randomly divided into 7 treatment groups, with 4 mice in each group. The treatment of each group was as follows: figure 1 :
[0097] Ctr group: drink water and food freely, orally orally gavage 200 μL PBS;
[0098] 50mg / kg PPT group: free to drink water, free to eat, daily oral administration of pomegranate peel tannin (PPT) with a concentration of 5mg / mL, dosage (μL)=mouse body weight (g)×10;
[0099] 100mg / kg PPT group: drink water freely, feed freely, and administer pomegranate peel tannin (PPT) with a concentration of 10mg / mL by oral gavage every day, and the administration dose (μL)=mouse body weight (g)×10;
[0100] 200mg / kg PPT group: drink water freely, feed fr...
Embodiment 3
[0110] Embodiment 3: the effect of pomegranate peel tannin on the mouse ulcerative colitis induced by dextran sulfate sodium salt (DSS)
[0111] The mouse ulcerative colitis model was established by induction with dextran sulfate sodium salt (DSS), and the therapeutic effect of pomegranate peel tannin (PPT) on the model was verified. The specific steps are as follows:
[0112] 1) The pomegranate peel tannin (PPT) prepared in Example 1 was prepared into a 40 mg / mL dilution with PBS buffer.
[0113] 2) Take 6-week-old C57 BL / 6 female mice with a body weight of 14-16 g and randomly divide them into 4 treatment groups with 7 rats in each group. The treatment of each group is as follows: Figure 5 :
[0114] Ctr group: free drinking water (without DSS), free intake of food, orally gavage 200 μL PBS;
[0115] PPT group: free drinking water (excluding DSS), free intake of food, daily oral gavage administration concentration is the pomegranate peel tannin (PPT) of 40mg / mL, administr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com