Tumor vaccine and preparation method thereof
A tumor vaccine and tumor technology, applied in the field of tumor vaccine and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The preparation of this application tumor vaccine
[0073] 1. Experimental Materials and Reagents
[0074] The C57BL / 6 mice used in the experiment were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18g.
[0075] Fetal calf serum (FCS): purchased from PAN company;
[0076] RPMI-1640 medium: purchased from PAN company;
[0077] GM-CSF and IL-4 were purchased from Peprotech, USA;
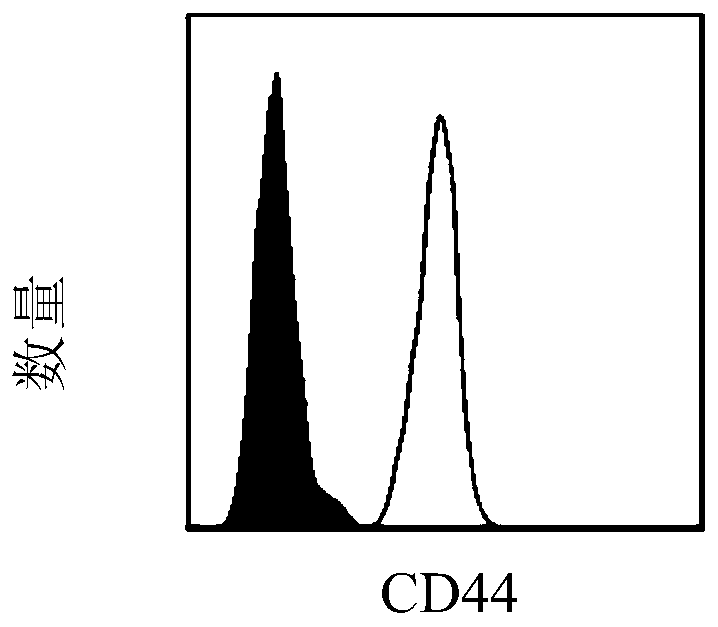
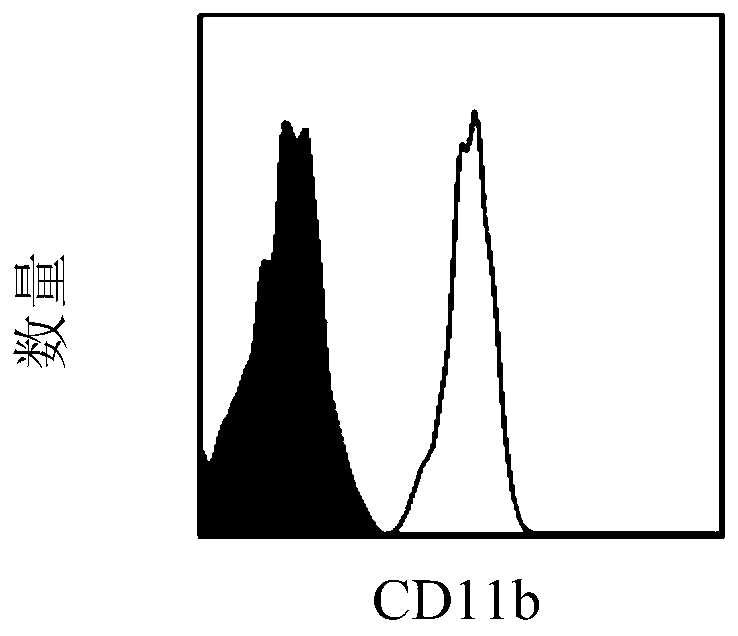
[0078] A lentiviral vector for simultaneously knocking out CD44 and CD11b genes: purchased from Beijing Hopson Genetics Company.
[0079] 2. Experimental steps
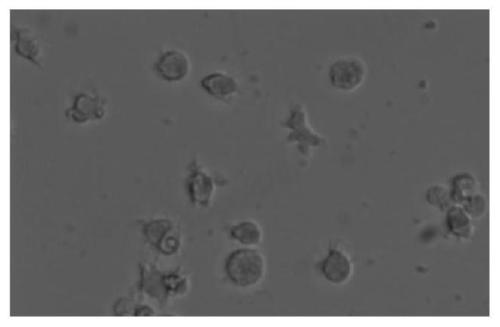
[0080] (1) Isolating mouse femur bone marrow hematopoietic precursor cells;
[0081] (2) The isolated mouse bone marrow hematopoietic precursor cells were mixed with 2×10 6 / mL density cultured in RPMI-1640 medium supplemented with 50ng / mL GM-CSF, 50ng / mL IL-4, 10% fetal calf serum (FCS), temperature 37°C, CO 2 The concentration is 5v / v%, induced for 7 days, the cell...
Embodiment 2
[0086] The preparation of the application tumor vaccine
[0087] 1. Experimental materials and reagents
[0088] The C57BL / 6 mice used in the experiment were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18g.
[0089] Fetal calf serum (FCS): purchased from PAN company;
[0090] RPMI-1640 medium: purchased from PAN company;
[0091] GM-CSF and IL-4 were purchased from Peprotech, USA;
[0092] Anti-mouse CD44 antibody and anti-mouse CD11b antibody were purchased from Biolegend, USA, the catalog number of anti-mouse CD44 antibody was 156002, and the catalog number of anti-mouse CD11b antibody was 101248.
[0093] 2. Experimental steps
[0094] (1) Isolating mouse femur bone marrow hematopoietic precursor cells;
[0095] (2) The isolated mouse bone marrow hematopoietic precursor cells were mixed with 2×10 6 / mL density cultured in RPMI-1640 medium supplemented with 50ng / mL GM-CSF, 50ng / mL IL-4, 10% FCS, temperat...
Embodiment 3
[0100] The tumor vaccine of the present application (that is, dendritic cells (CD11b knockout CD44 and CD11b gene) - / - CD44 - / - DC)) and conventional dendritic cell tumor vaccine (that is, mature dendritic cells (CD11b + / + CD44 + / + DC)) comparison of the ability to activate the immune system of the recipient.
[0101] 1. Experimental materials and reagents
[0102] The C57BL / 6 mice used in the experiment were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., aged 6-8 weeks, weighing about 18g.
[0103] Cytokines in serum were detected using Biolegend Cytokine Detection Kit (Cat. No. 740005).
[0104] anti-mouse H 2 K b ,Ia b , CD40, CD80, CD86, CD44, and CD11b fluorescent antibodies were purchased from Biolegend Company in the United States, and the article numbers were H 2 K b (114605), Ia b (116410), CD40 (124607), CD80 (104705), CD86 (105005), CD44 (156002), CD11b (101248).
[0105] The antibody used to label mouse peripheral blood CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com