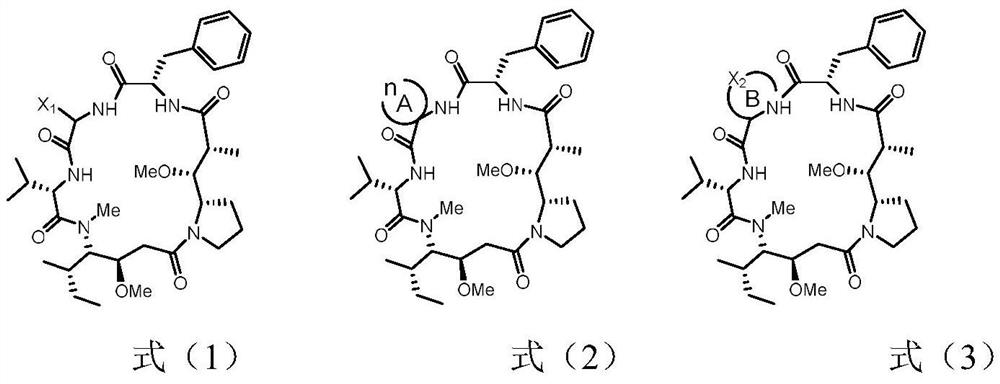
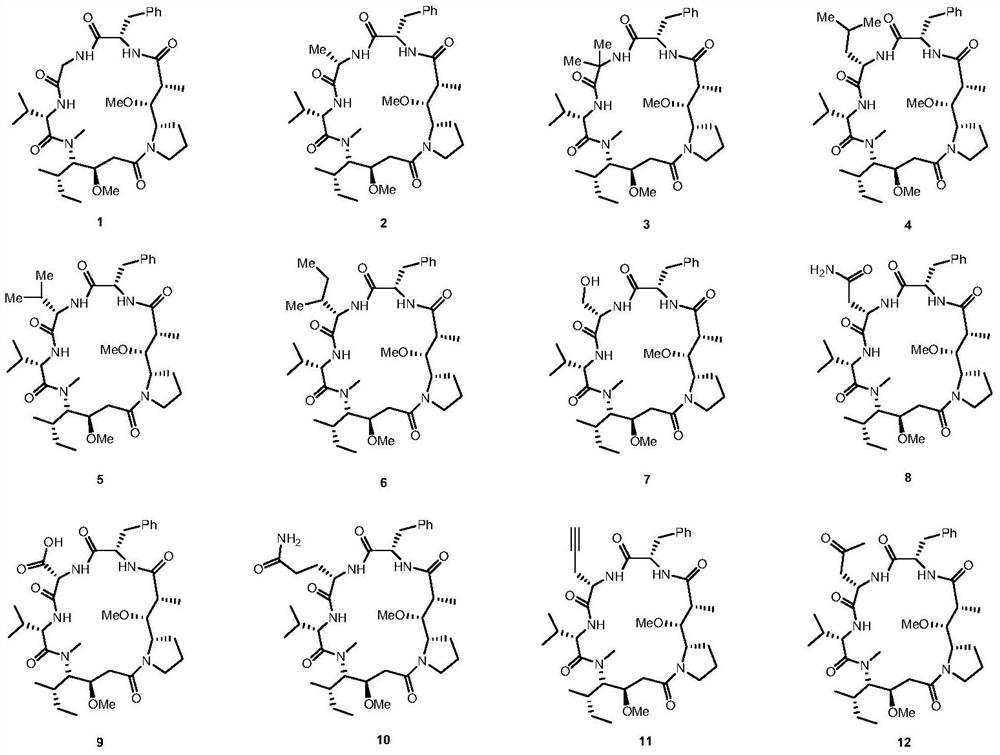
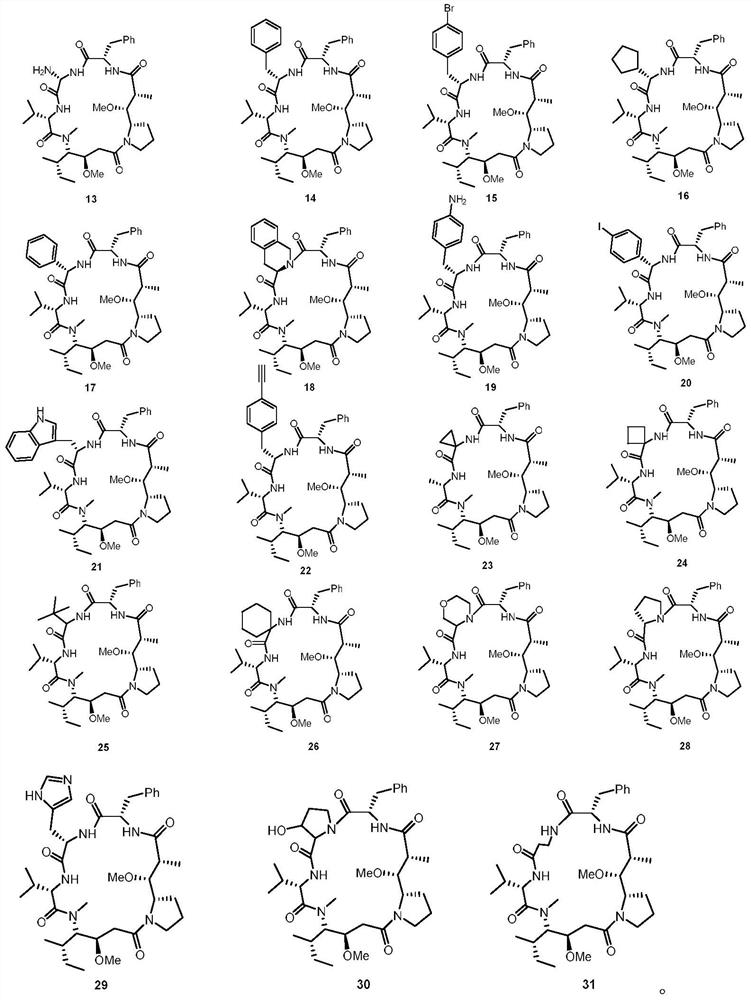
Dolastatin 10-cyclic peptide derivative and its preparation method and application
A technology of dolastatin and derivatives, applied in cyclic peptide components, peptides, drug combinations, etc., can solve the problems of increasing compound structure, high toxicity, poor stability, etc., and achieve the effects of low toxicity, low cost and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Compound 1
[0040]
[0041] Weigh the linear pentapeptide and dissolve it in DMF (1×10 -3 mol / L), cooled in an ice-water bath, protected by nitrogen, then added DMAP (2 equiv.) in turn, kept stirring in the ice-water bath for 5 min after the addition, then added EDCI (5 equiv.) at one time, and continued to maintain the ice-water bath reaction for 1-2 hours, then the temperature was raised to room temperature, and the reaction was carried out for about 6 hours until the reaction was completed by LC-MS detection. Water was added to quench, extracted with ethyl acetate, the organic phase was washed with 10% aqueous citric acid solution, water, saturated brine, and anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain a crude product of cyclic peptide. The crude product was separated and purified by preparative HPLC to give cyclic peptide compound 1 as a white solid (10 mg, 47%). 1 H NMR (400MHz, DMSO-d 6)δ7.41–7.05(m...
Embodiment 2
[0042] Example 2 Compound 2
[0043]
[0044] The operation procedure was the same as that of Example 1, white solid (10 mg, 35%). 1 H NMR (400MHz, DMSO-d 6 )δ7.24(s,5H),5.37(dd,J=11.9,7.8Hz,2H),4.89–4.75(m,1H),4.68(s,1H),4.52–4.39(m,1H),4.34 –4.22(m, 1H), 4.05(s, 1H), 3.72 – 3.54(m, 2H), 3.23(s, 3H), 3.07(s, 2H), 2.95 – 2.73(m, 2H), 2.56(s ,4H),2.45(dd,J=31.6,12.4Hz,2H),2.31-2.16(m,2H),2.06(dd,J=15.2,7.2Hz,6H),1.80-1.66(m,2H), 1.54–1.46 (m, 3H), 1.12 (d, J=4.4Hz, 4H), 1.01–0.87 (m, 13H). HRMS (ESI; m / z) [M+Na] + calcd for C 35 H 57 N 5 O 7 Na 694.4156, found 694.4106.
Embodiment 3
[0045] Example 3 Compound 3
[0046]
[0047] The operation procedure was the same as that of Example 1, white solid (13 mg, 41%). 1 H NMR (400MHz, DMSO-d 6 )δ7.41–7.11(m,5H),4.54(ddd,J=28.1,14.9,8.3Hz,1H),4.32–4.20(m,1H),4.19–4.09(m,1H),4.08–4.00( m, 1H), 3.66 (dd, J=12.2, 6.0Hz, 1H), 3.48 (t, J=7.7Hz, 1H), 3.29 (t, J=5.1Hz, 3H), 3.20–3.14 (m, 2H) ), 3.13 (s, 2H), 2.92 (dd, J=12.2, 6.1Hz, 1H), 2.87–2.77 (m, 1H), 2.33 (ddd, J=17.8, 11.9, 5.6Hz, 1H), 2.21– 2.06(m, 2H), 2.05-1.84(m, 4H), 1.68(dt, J=15.0, 12.8Hz, 2H), 1.53-1.39(m, 2H), 1.34(s, 2H), 1.26(d, J=22.0Hz,8H),1.08–0.75(m,17H).HRMS(ESI;m / z)[M+H] + calcd for C 37 H 60 N 5 O 7 686.4493, found 686.4545.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com