Antibody-mediated autocatalytic, targeted delivery of nanocarriers to tumors
A nanocarrier and antibody technology, applied in antitumor drugs, drug delivery, powder delivery, etc., can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0321] The first is through the incorporation of stabilizers with chemical functional groups during the preparation of the particles, for example during the emulsion preparation of the particles.
[0322] The second is by direct crosslinking of particles and ligands with homobifunctional or heterobifunctional crosslinkers after particle preparation. This second procedure can use an appropriate chemistry and a class of cross-linking agents (CDI, EDAC, glutaraldehyde, etc., as discussed in more detail below) or any other agent that enables the ligand to be modified by chemical modification of the particle surface after preparation. A crosslinker coupled to the particle surface. This second category also includes a method in which amphiphilic molecules, such as fatty acids, lipids, or functional stabilizers, can be passively adsorbed and adhered to particle surfaces, thereby introducing functional end groups attached to ligands.
[0323] Various other methods of manufacturing ac...
example 1
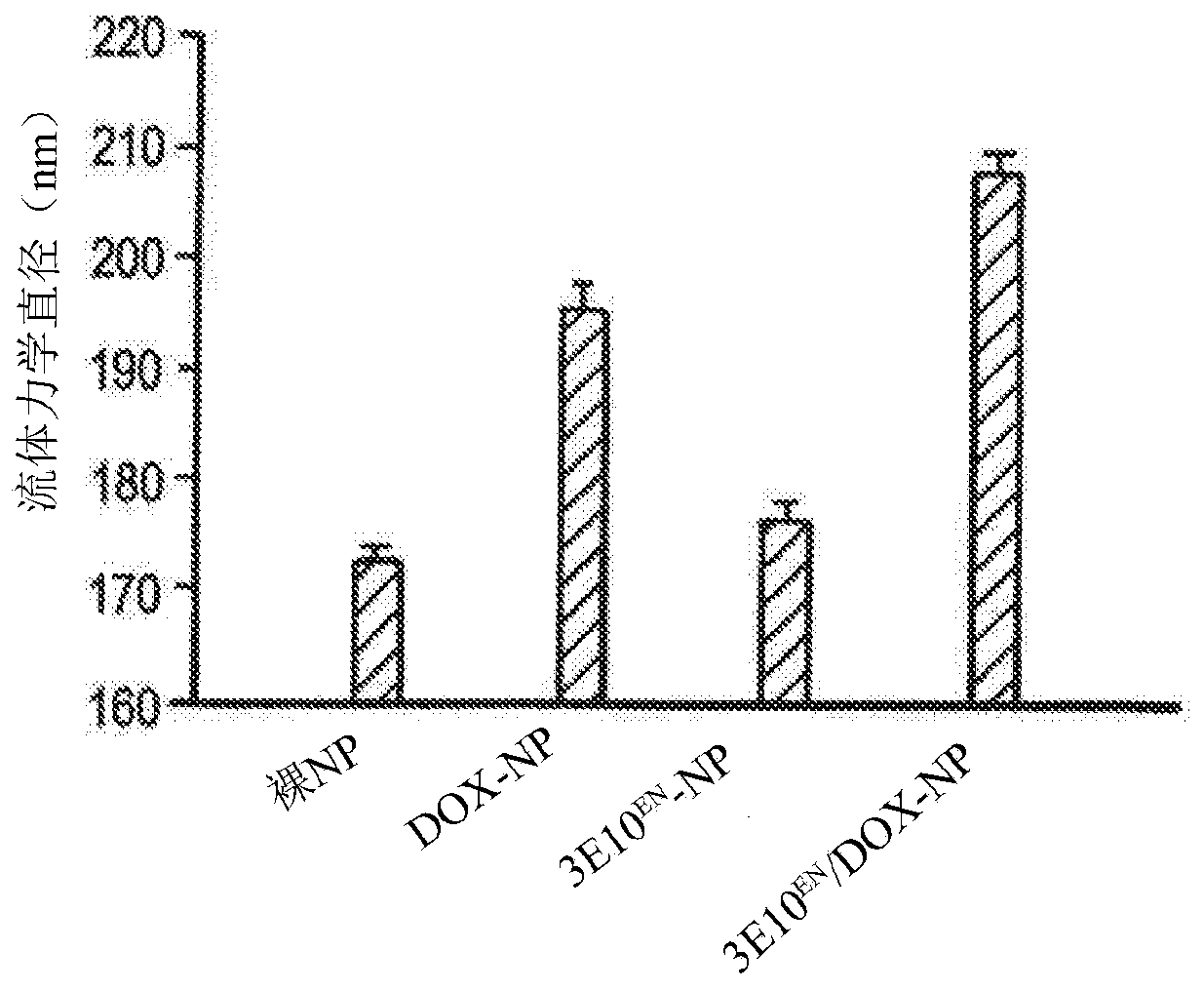
[0417] Example 1: Synthesis and characterization of nanoparticles with and without surface anti-DNA autoantibodies
[0418] Materials and methods
[0419] Materials and Cell Culture
[0420] All chemicals were purchased from Sigma-Aldrich unless otherwise stated. The mouse mammary tumor cell line 4T1 was obtained from the American Type Culture Collection (ATCC) and incubated in a 37°C incubator with 5% CO2 supplemented with 10% fetal bovine serum (Invitrogen). , 100 units / ml penicillin and 100 μg / ml streptomycin (Invitrogen) in DMEM medium (Invitrogen).
[0421] Synthesis of PLGA-PLL
[0422] PLGA-PLL was synthesized according to previously reported procedures (Zhou et al., Biomaterials, 33(2):583-591 (2012); Han et al., Nanomedicine (2016)). Briefly, PLGA (3 g, 50:50 PLGA acid end groups; i.v. about 0.67 dL / g; Absorbable Polymers, AL) and 200 mg poly(ε- Benzyloxycarbonyl-L-lysine) (PLL) (MW 1000-4000 Da, Sigma) was dissolved in 6 mL of dimethylformamide in a dry round bo...
example 2
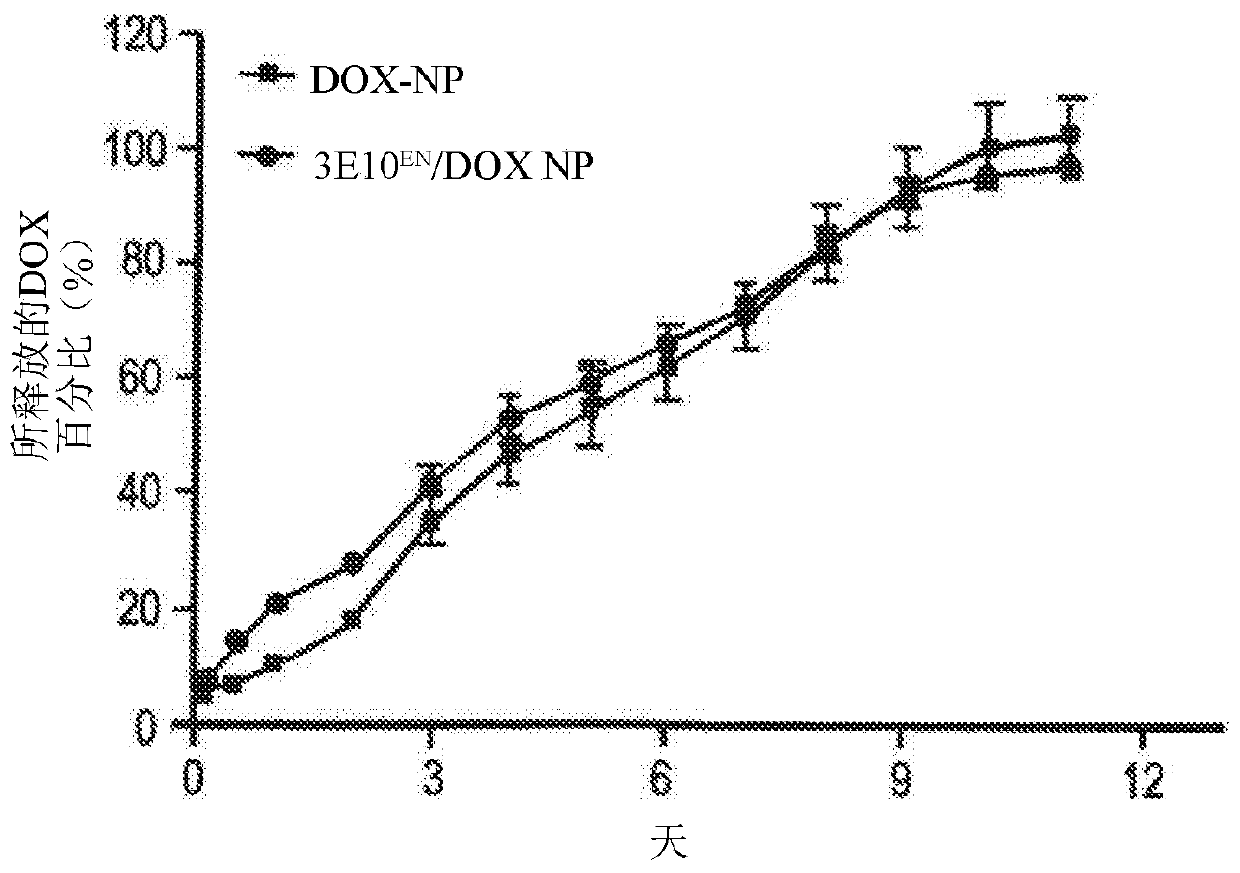
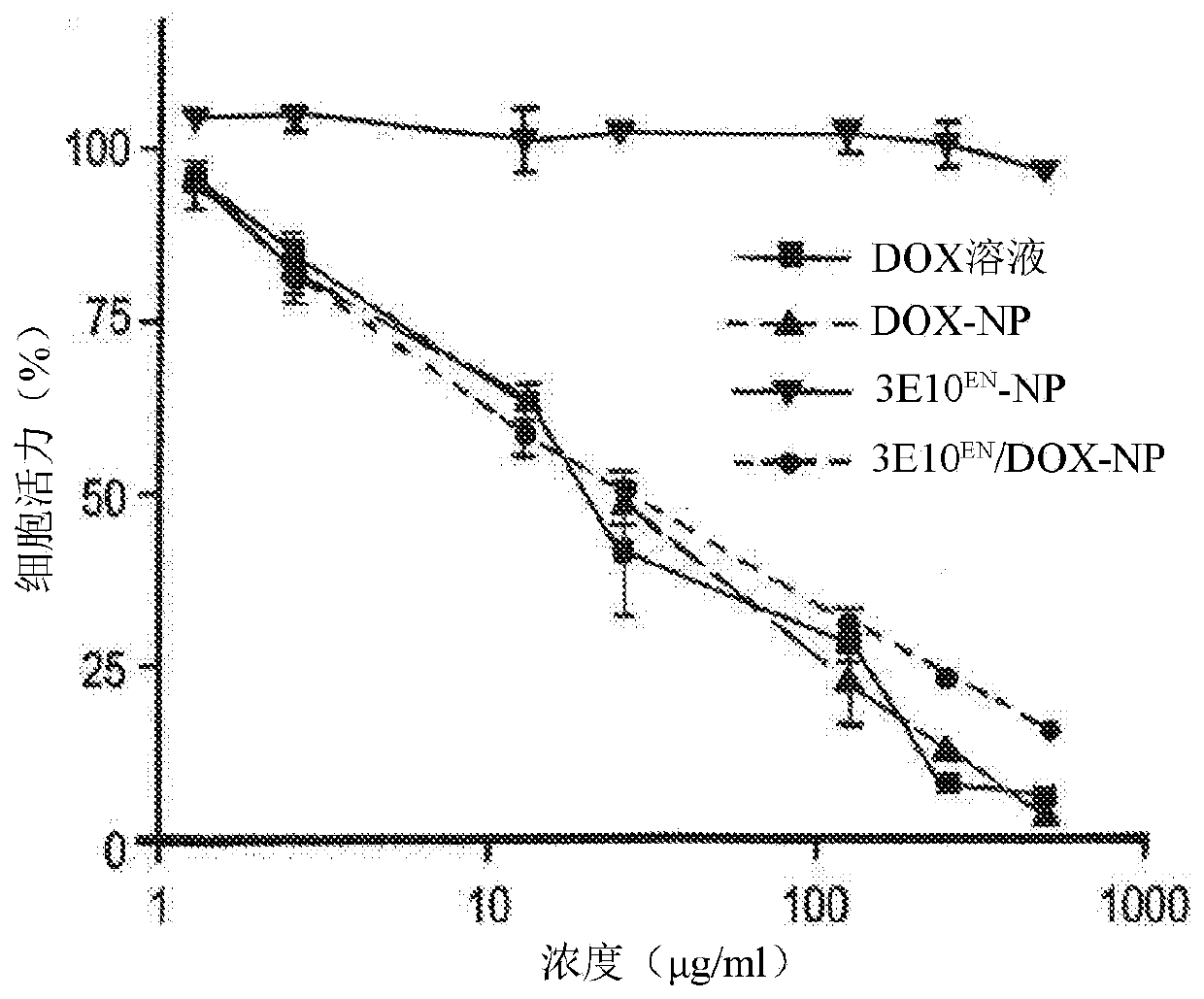
[0448] Example 2: 3E10 EN Conjugation enhances the interaction of nanoparticles with exDNA
[0449] Materials and methods
[0450] DNA-binding ability of 3E10-conjugated nanoparticles
[0451] Plasmid DNA pGL4.74 (20 μg, Promega) was linearized using BamHI to expose the phosphate group, which was then replaced with N-(3-dimethylaminopropyl)-N'-ethylcarbodiethylene Amine hydrochloride (EDC, 0.20 mg, 1.0 μmol) and N-hydroxysuccinimide (NHS, 0.17 mg, 1.5 μmol) were activated and reacted with NH2-PEG50-NH2 (0.41 mg, 3.0 μmol). Amino-terminated DNA was then thiolated with Trout's reagent (0.68 mg, 5.0 μmol) and coated onto the surface of a glass plate functionalized with maleimide groups (MicroSurfaes). To determine the binding capacity of the nanoparticles, with and without 3E10 EN The IR780-loaded nanoparticles were resuspended in PBS at 1 mg / ml and added to the surface of a glass plate. After incubation for 1 hour at room temperature, the glass plate was rinsed with water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com