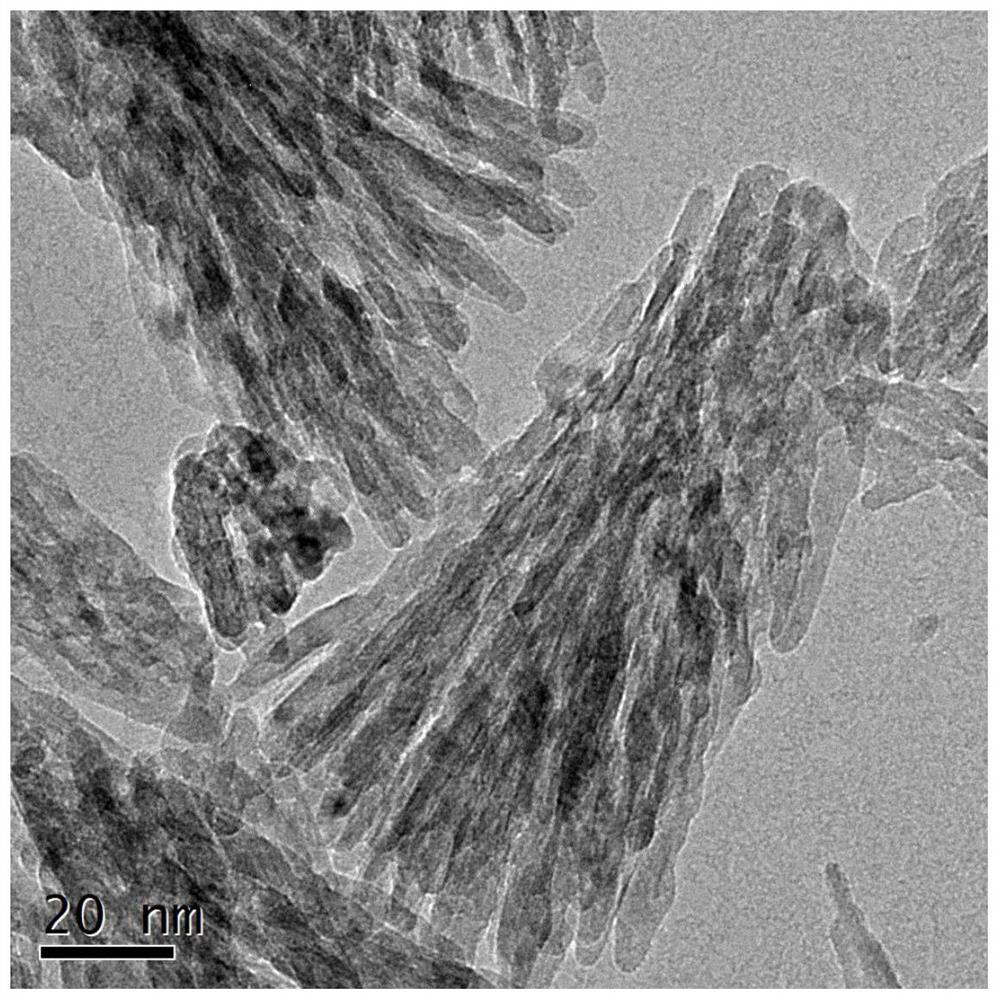
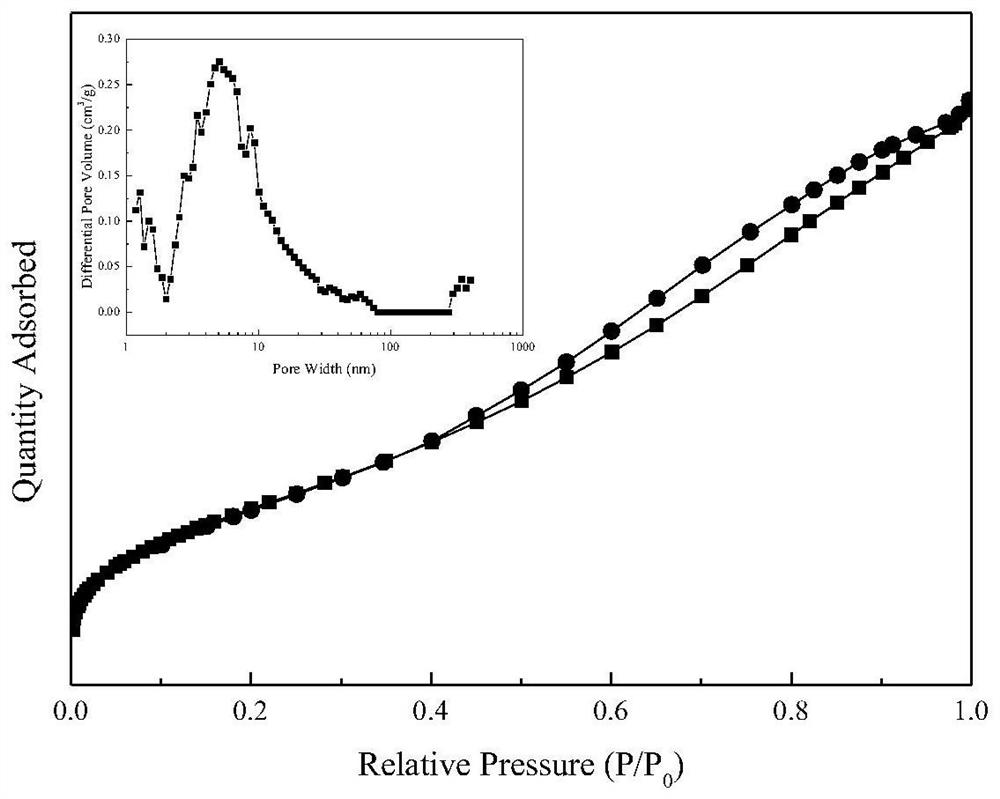
A highly stable catalyst for hydrogen chloride oxidation to chlorine
A high-stability, oxidation-based technology, used in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chlorine/hydrogen chloride, etc. The effect of sintering deactivation and enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Place a beaker containing 100 mL of deionized water in an ice-water bath, and continue magnetic stirring, and then drop 10 mL of TiCl 4 , a clear and transparent solution was obtained. 1.25 g of 4-hydroxypiperidine and 5.00 g of cetyl ammonium sulfate were added to the resulting solution and stirring was continued for 1 h. The resulting mixture was sealed and kept in a water bath at 50°C for 24 hours, then filtered, washed with water and alcohol three times each, dried at 50°C for 12 hours, and then calcined in an air atmosphere at 450°C for 4 hours to obtain 6.51 g of the prepared TiO 2 , denoted as TiO 2 -1. The target amount was obtained by accumulating multiple times through the same preparation method.
[0031] Add 2.0g RuCl 3 ·3H 2 O was dissolved in 400mL deionized water, and the second component 4.5g Ce(NO 3 ) 3 ·6H 2 O, 1.5g ZrOCl 3 ·8H2 O, add 100g TiO 2 -1, vigorously stirred, impregnated for 24 hours; centrifuge the obtained material, wash it full...
Embodiment 2
[0033] TiO 2 -1 is prepared with embodiment 1. Add 3.2g of anhydrous ruthenium trichloride to 500mL of deionized water, fully dissolve, then add the second component SnCl 4 ·5H 2 O 5.4g, 150g TiO 2 -1 was added and stirred vigorously, impregnated for 12 hours; then the obtained material was centrifuged, washed fully with deionized water, then dried at 80°C, granulated, shaped and dried in air atmosphere, at 350°C After roasting for 6 hours, the catalyst B for producing chlorine by catalytic oxidation of hydrogen chloride is obtained.
Embodiment 3
[0035] Place a beaker containing 200 mL of deionized water in an ice-water bath, and continue magnetic stirring, and then drop 15 mL of TiCl 4 , a clear and transparent solution was obtained. 7.20 g of ammonium lauryl sulfate and 3.54 g of triethylenediamine were added to the resulting solution, and stirring was continued for 1 h. The resulting mixture was sealed and kept in a water bath at 50°C for 18 hours, then filtered, washed with water and alcohol three times each, dried at 50°C for 12 hours, and then calcined in air at 400°C for 4 hours to obtain 9.85 g of the prepared TiO 2 , denoted as TiO 2 -2. The target amount was obtained by accumulating multiple times through the same preparation method.
[0036] Add 1.2g RuCl 3 ·3H 2 O was added to 400mL deionized water, and then the second component SmCl was added 3 ·6H 2 O2.1g, SnCl 4 ·5H 2 O 3.6g, TiO 2 -2 Add to the above solution and stir vigorously, impregnate for 20 hours; then centrifuge the obtained material,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com