Traditional Chinese medicine composition, preparation method and application in preparing drug for treating chronic non-atrophic gastritis
A composition and technology of traditional Chinese medicine, applied in the field of traditional Chinese medicine composition, preparation and treatment of chronic non-atrophic gastritis, can solve the problems of untimely treatment, affecting the quality of life of patients, long course of disease, etc., and achieve inhibition of gram-positive bacteria, hemostasis Fast and sure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
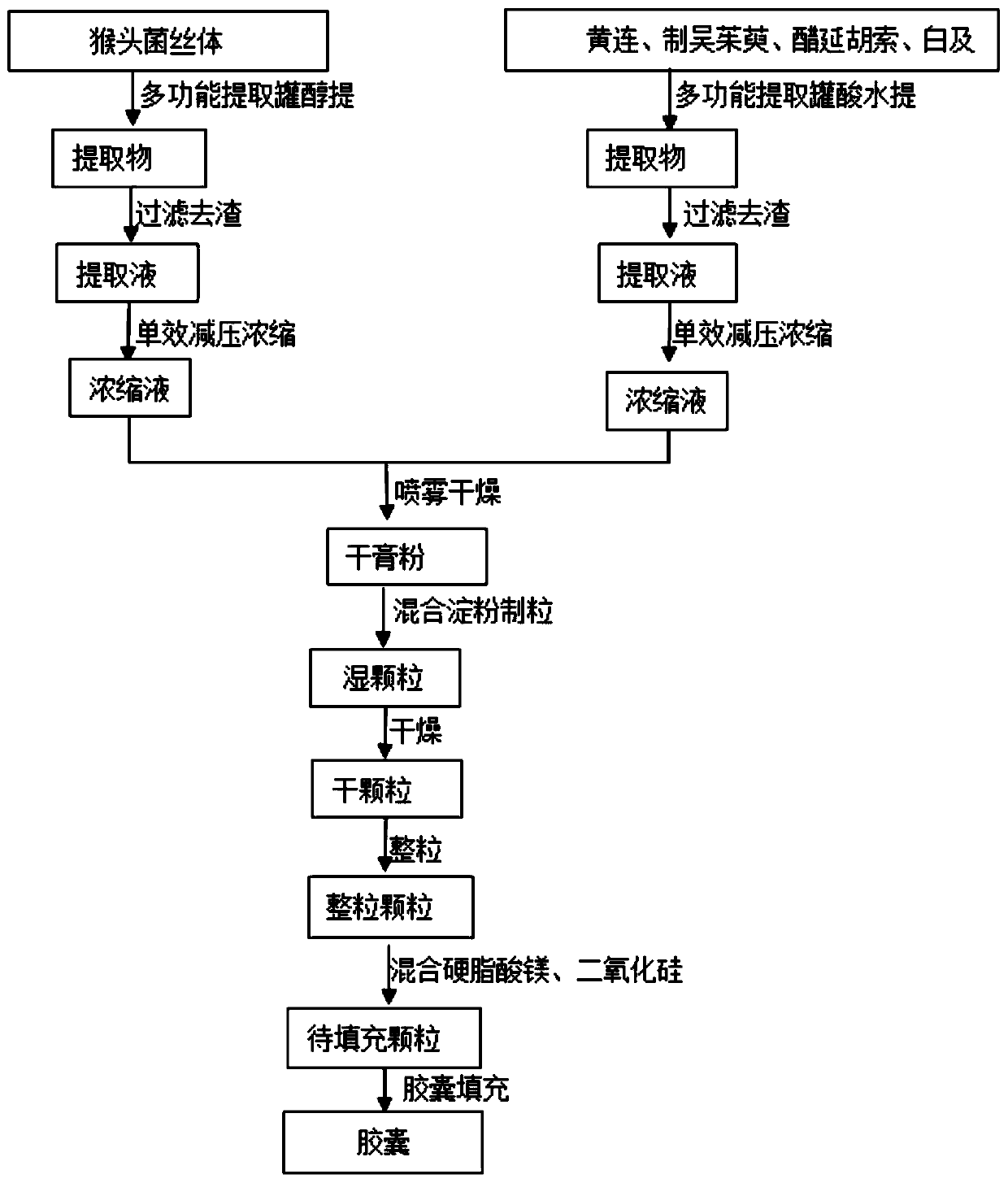
[0023] The content of the present invention includes the following components in parts by weight: 3 parts of Hericium erinaceus mycelium, 3 parts of Coptidis rhizome, 1 part of Evodia rutaecarpa, 3 parts of vinegar corydalis and 1 part of Baiji.
[0024] Take Hericium erinaceus, add volume concentration and be 60% ethanol to extract three times, add 6 times of 60% ethanol for the first time, soak for 0.5 hour, extract for 2 hours, add 4 times of 60% ethanol for the second time, extract for 1.5 hours , add 4 times the amount of 60% ethanol for the third time, extract for 1.5 hours, filter, combine the extracts, reclaim the ethanol, concentrate under reduced pressure to a relative density of 1.10~1.15 (60°C), to obtain Hericium erinaceus mycelium fluid extract, spare. For the rest of Coptis chinensis, Evodia rutaecarpa, Vinegar Corydalis Corydalis, Bai and four medicinal materials, add acid water (glacial acetic acid to adjust the pH to 3.0) and decoct three times. Add 6 times t...
Embodiment 2
[0029] Take Hericium erinaceus, add volume concentration and be 50% ethanol to extract three times, add 6 times of 50% ethanol for the first time, soak for 0.5 hour, extract for 2 hours, add 4 times of 50% ethanol for the second time, extract for 1.5 hours , add 4 times the amount of 50% ethanol for the third time, extract for 1.5 hours, filter, combine the extracts, reclaim the ethanol, concentrate under reduced pressure to a relative density of 1.10~1.15 (60°C), and obtain Hericium erinaceus mycelium fluid extract, spare. For the rest of Coptis chinensis, Evodia rutaecarpa, Vinegar Corydalis Corydalis, Bai and four medicinal materials, add acid water (glacial acetic acid to adjust the pH to 4.0) and decoct three times. For the first time, add 7 times the amount of acid water to soak for 0.5 hours, decoct for 2 hours, and then decoct for 2 hours. Add 5 times the amount of acid water three times, decoct for 1.5 hours respectively, combine the extracts, concentrate under reduce...
Embodiment 3
[0031] Analysis and Evaluation of Effectiveness Research Results
[0032] Control group: patent application number 201310013628.0 (a medicinal composition for stomach diseases against Helicobacter pylori).
[0033] (1) Anti-Helicobacter pylori (Hp) test of this application: It is divided into two parts: in vitro antibacterial test and in vivo effect test on Hp infection-induced chronic non-atrophic gastritis model in rats:
[0034] ① In vitro antibacterial test: routinely prepare Hp bacterial liquid (standard strain ATCC43504), culture and count viable bacteria after culturing at 37°C for 7 days, and adjust the bacterial concentration to 1×10 8 CFU / mL was prepared as the parent bacterial suspension and stored at 4°C for future use. During the test, the mother bacteria solution was taken to adjust the bacterial concentration to 2×10 7 CFU / mL is used as the working bacterial suspension. Accurately weigh an appropriate amount of the dry extract of this application, dissolve it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com