Preparation method of glimepiride intermediate sulfonamides analogue 1 and the sulfonamide analogue 2
A technology for sulfonamides and analogs, which is applied in the field of preparation of sulfonamide analog 1 and sulfonamide analog 2, the key intermediates of glimepiride, can solve problems such as no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
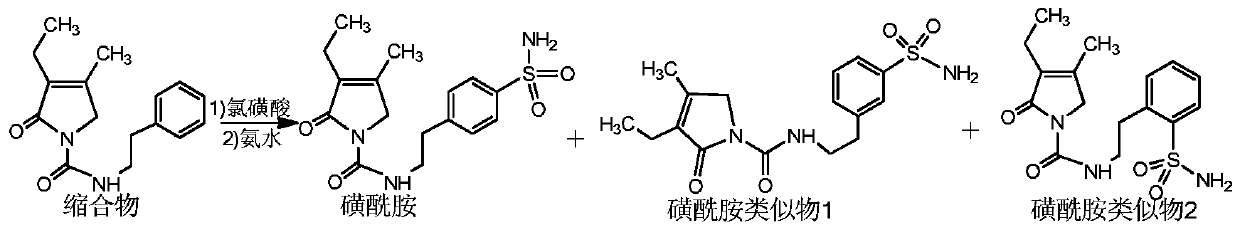
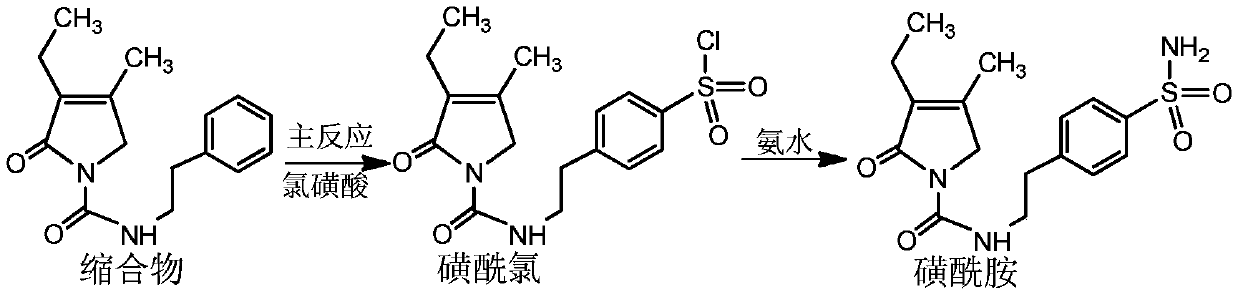
[0032] Add 150g of chlorosulfonic acid to a 250ml reaction bottle, cool down to below 20°C, slowly add 30g of condensate at 10-20°C, then raise the temperature to 35°C, and react at 35±2°C for 5 hours. After the reaction was completed, the reaction solution was slowly poured into 1000 ml of ice water. Then filter and wash with water to obtain a white solid.
[0033] Add the white solid to a 100ml reaction flask, add 800ml of concentrated ammonia water, react at 20-25°C for 4 hours, then raise the temperature to 40±2°C, and continue the reaction for 4 hours. After the reaction, the temperature was lowered to below 25°C, filtered and washed with water to obtain a white solid.
[0034] Finally, the white solid was preparatively chromatographed by HPLC, chromatographic conditions: chromatographic column: C18, 300*50.0mm, particle size 10μm, pore size 120A, wavelength: 225nm, mobile phase A: methanol, mobile phase B: water, isocratic washing A 80% and B 20% (V / V) were separated t...
Embodiment 2
[0036] Add 180g of chlorosulfonic acid into a 250ml reaction bottle, cool down to below 20°C, slowly add 30g of condensate at 10-20°C, then raise the temperature to 40°C, and react at 40±2°C for 7 hours. After the reaction was completed, the reaction solution was slowly poured into 1000 ml of ice water. Then filter and wash with water to obtain a white solid.
[0037] Add the white solid to a 100ml reaction flask, add 800ml of concentrated ammonia water, react at 20-25°C for 4 hours, then raise the temperature to 40±2°C, and continue the reaction for 4 hours. After the reaction, the temperature was lowered to below 25°C, filtered and washed with water to obtain a white solid.
[0038] Finally, the white solid was preparatively chromatographed by HPLC, chromatographic conditions: chromatographic column: C18, 300*50.0mm, particle size 10μm, pore size 120A, wavelength: 225nm, mobile phase A: methanol, mobile phase B: water, isocratic washing A 80% and B 20% (V / V) were separated...
Embodiment 3
[0040] Add 300g of chlorosulfonic acid into a 250ml reaction bottle, cool down to below 20°C, slowly add 30g of condensate at 10-20°C, then raise the temperature to 50°C, and react at 50±2°C for 8 hours. After the reaction was completed, the reaction solution was slowly poured into 1000 ml of ice water. Then filter and wash with water to obtain a white solid.
[0041] Add the white solid to a 100ml reaction flask, add 800ml of concentrated ammonia water, react at 20-25°C for 4 hours, then raise the temperature to 40±2°C, and continue the reaction for 4 hours. After the reaction, the temperature was lowered to below 25°C, filtered and washed with water to obtain a white solid.
[0042] Finally, the white solid was preparatively chromatographed by HPLC, chromatographic conditions: chromatographic column: C18, 300*50.0mm, particle size 10μm, pore size 120A, wavelength: 225nm, mobile phase A: methanol, mobile phase B: water, isocratic washing A 80% and B 20% (V / V) were separated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com