Application of palbociclib in mucosal malignant melanoma
A malignant melanoma, mucosal technology, applied in the field of biomedicine, can solve the problems of limited promotion and application, and can not improve the five-year survival rate of patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
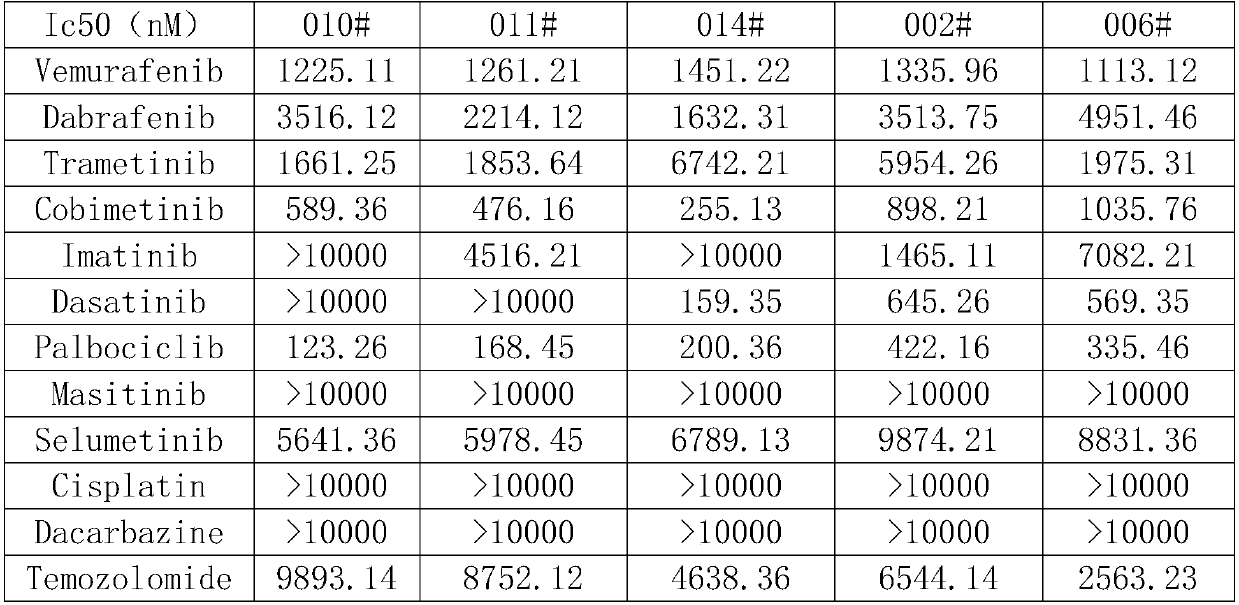
[0079] Example 1 PDC (patient derived cell) screening sensitive candidate compounds
[0080] Inclusion criteria: Primary lesions of malignant melanoma occurring in the mucosa. There is no clear classification of mucosal malignant melanoma. Staging according to AJCC.
[0081] The obtained mucosal malignant melanoma samples were subjected to PDC model screening for sensitive inhibitors, as follows:
[0082] 1) Collect the PDC cells in the logarithmic growth phase, trypsinize, prepare the cell suspension, count the cells, and adjust the cell concentration to 2×10 4 a / ml;
[0083] 2) Inoculate the cells into a 96-well plate, 100ul of cell suspension per well, and set at least 3 auxiliary wells for each drug concentration;
[0084] 3) Place the cells in a 37°C, 5% carbon dioxide incubator and culture overnight;
[0085] 4) The next day after cell inoculation, according to the drug concentration gradient 0nM, 10 -3 nM, 10 -2 nM, 10 -1 nM, 1nM, 10nM, 100nM, 1000nM, 10uM, adde...
Embodiment 2
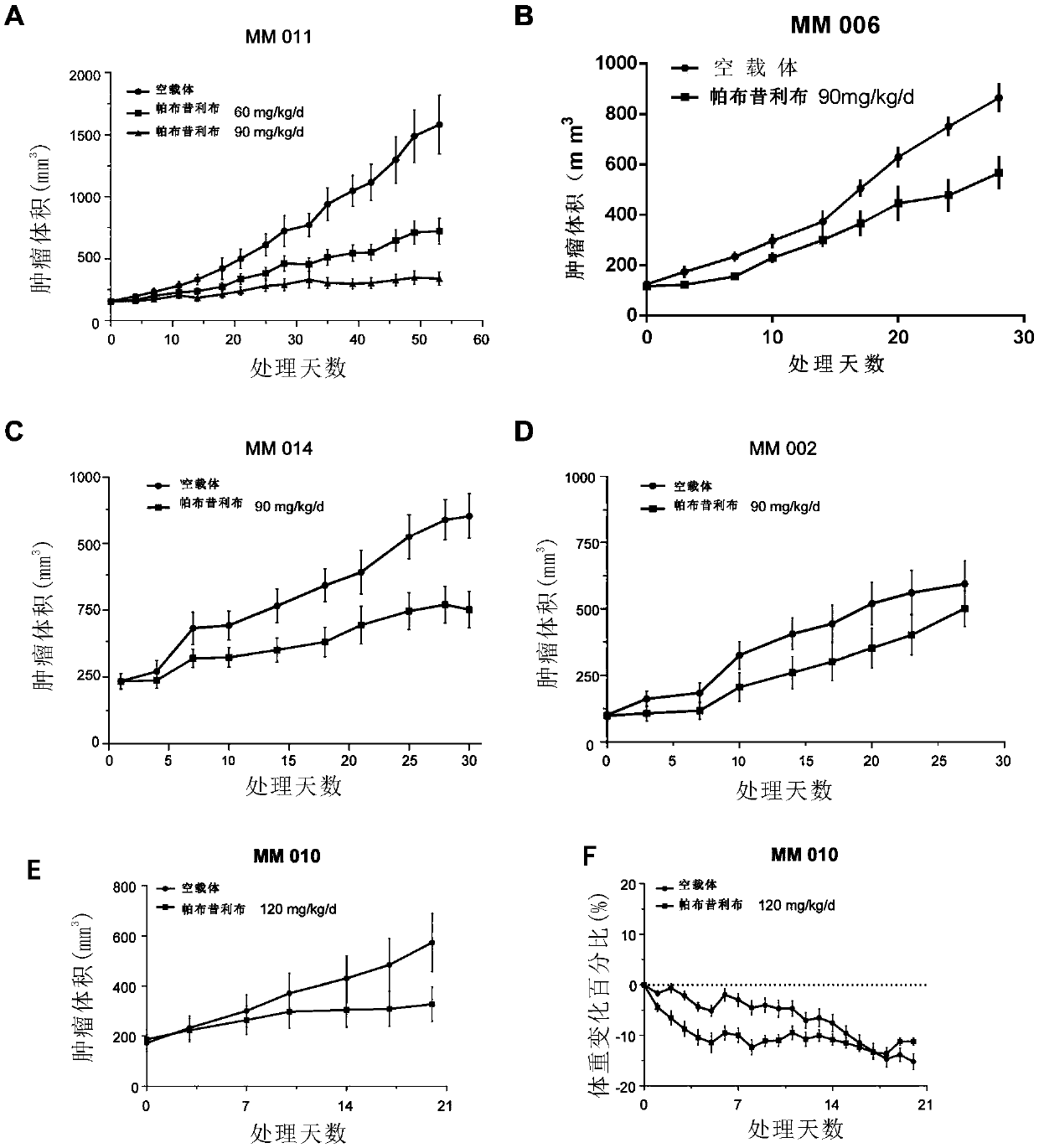
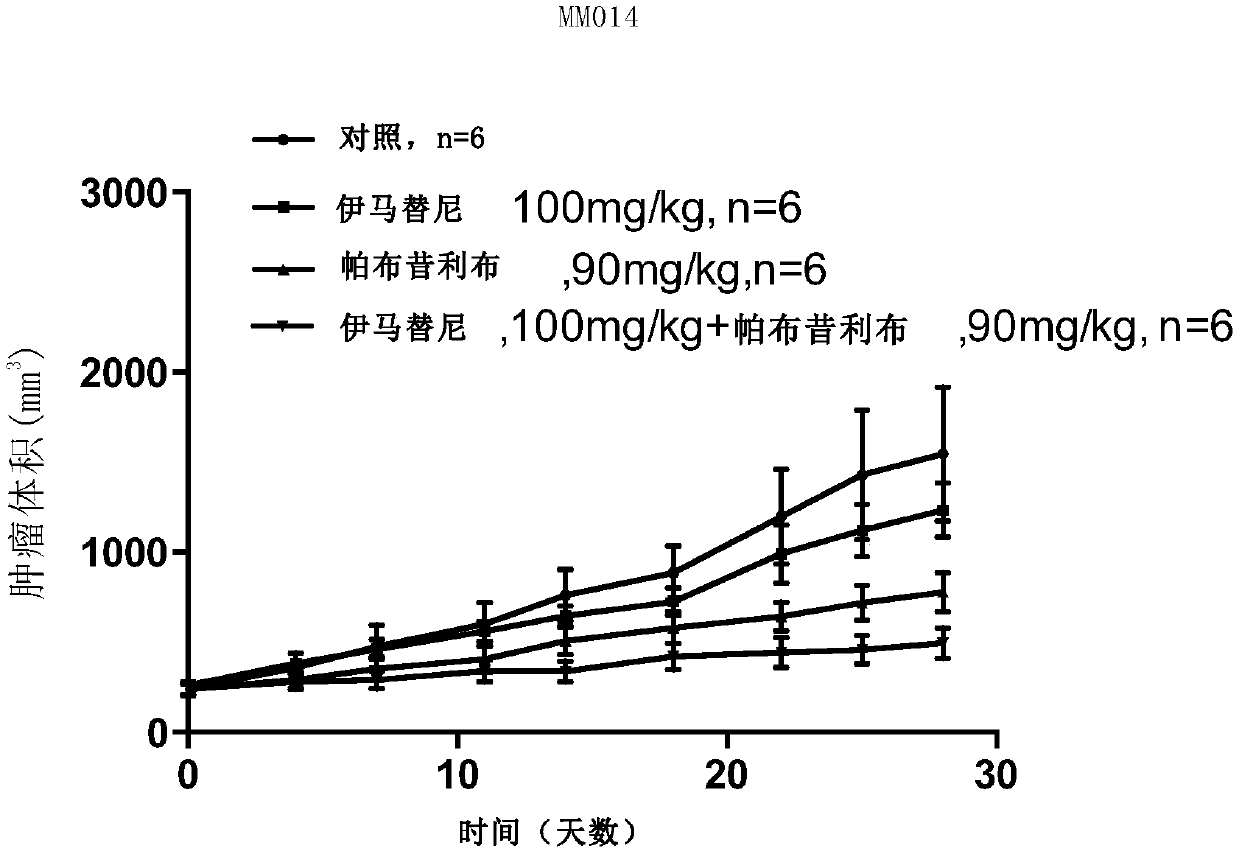
[0115] Example 2 Construction of xenograft tumor model and tumor suppressive effect of palbociclib
[0116] In order to better restore the biological and genetic characteristics of the patient's tissue-derived tumor, the samples collected in Example 1 were also used to construct a mouse xenograft tumor model, and the inhibitory effect of palbociclib was further verified.
[0117] 1) Put the sterile petri dish and surgical instrument package into the transfer bin for ultraviolet disinfection for 30 minutes;
[0118] 2) Weigh the mice, extract 10% chloral hydrate with a sterile syringe, calculate the dose according to the weight of the mice at 0.004ml / g, the maximum dose is not more than 0.1ml, and inject it into the abdominal cavity of the mice.
[0119] Precautions for anesthesia: Inject slowly, observe muscle tension at the same time, and the anesthesia is successful when the activity is obviously weakened or disappears;
[0120] After anesthesia, attention should be paid to...
Embodiment 3
[0129] Example 3 Whole Genome Sequencing
[0130] Whole-genome sequencing was performed on the five cell lines screened in Example 1, and it was found that in these mucosal malignant melanomas, CDK4, and well-known tumor driver genes such as TERT, MDM2, FRS2, KIT, BRAF, and CCND1 were significantly amplified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com