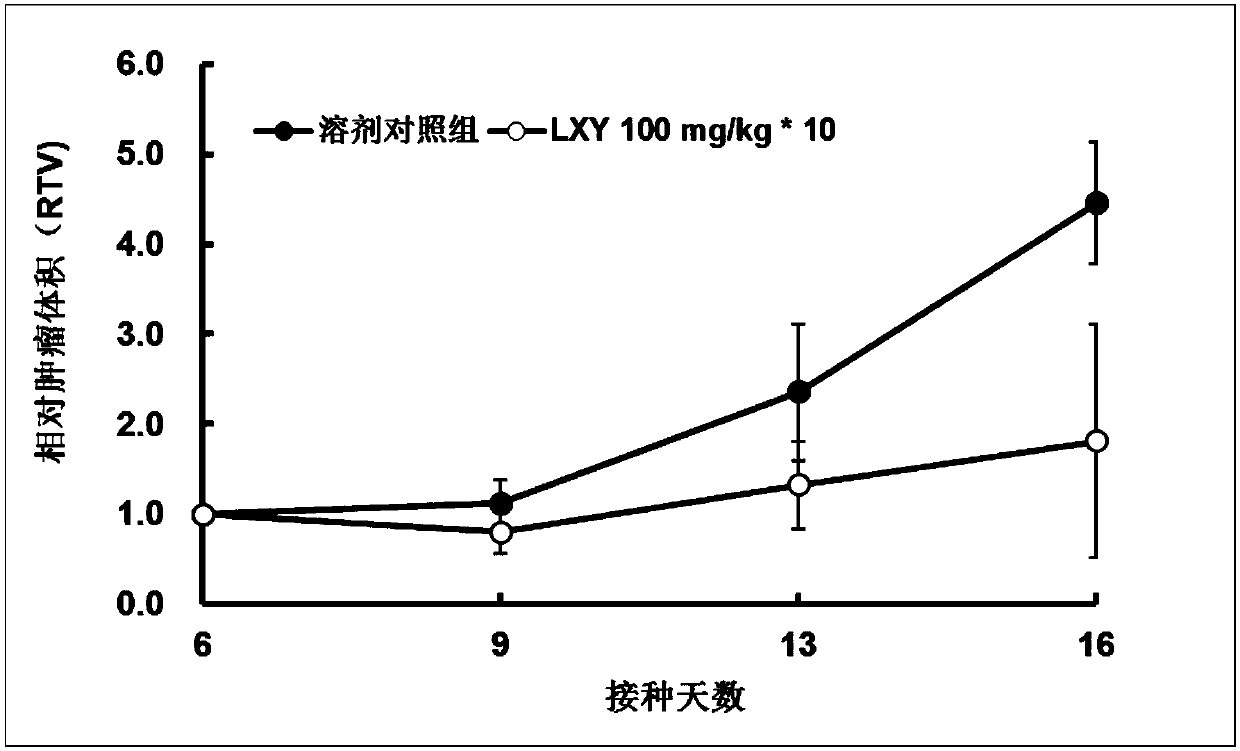
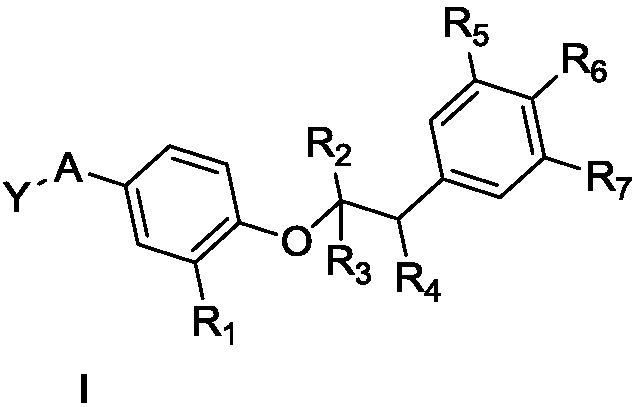
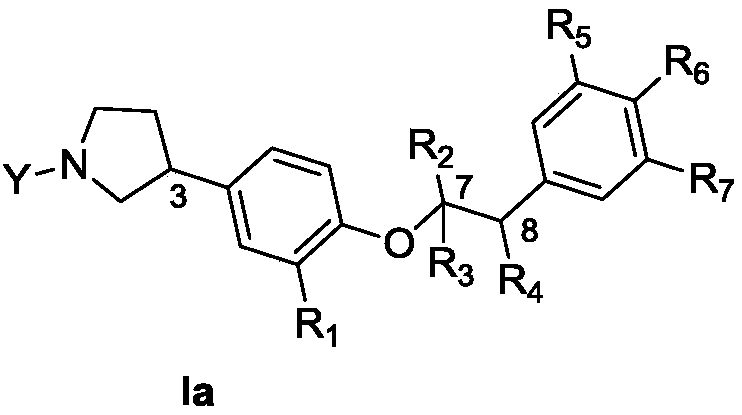
Structural simplification of natural product 4-O-methyl saucerneol of lignans, and preparation method, pharmaceutical composition and application thereof
A compound, methoxyl technology, applied in the field of medicine, can solve the problems of difficult extraction and separation, complex structure of natural products, limited sources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] 500mL round bottom bottle contains (25g, 0.137mol) homovanillic acid, 150ml DMF is dissolved, (66g, 0.477 mol) anhydrous K 2 CO 3 Add, add BnBr (34mL, 0.286mol) dropwise at room temperature, TLC shows complete after 5h, add 500mL EA, 500mL water to the reaction solution, separate, take the organic phase, extract the aqueous phase with EA (100ml*2), combine the EA The phases were washed with 200 mL of water and saturated sodium chloride successively, dried with anhydrous sodium sulfate, filtered, concentrated, and column chromatography (PE:EA=20:1) to obtain 42 g of the product with a yield of 85%.
[0119] (34g, 90mmol) of the above product in anhydrous methanol, the suspension was stirred for 5 minutes and then (30g, 540mmol) KOH was added, the reaction solution was clear, and the TLC was completed by stirring at room temperature for 4 hours. Concentrate the reaction solution, remove methanol, add 300mL water and 200mL ether to the reaction solution, separate the water pha...
Embodiment 2
[0122] Step A: A 200ml round-bottomed bottle contains (9.6g, 35.3mmol) of the above product, 20mL SOCl 2 Add, react at 55°C for 1.5h, concentrate, and set aside -I.
[0123] Add (6.43g, 36.4mmol) (S)-benzyloxazolidinone to a 250mL two-necked flask, dissolve in 100mL anhydrous THF, and drop (2.5M, 14.5mL, 36.4mmol) under the protection of argon at -78℃n -BuLi, drip for about 15min, react at this temperature for 0.5h, then drip the prepared I 10mL anhydrous THF solution, warm to room temperature and react for 3h to complete the reaction. 20mL saturated ammonium chloride was slowly dropped into the reaction solution, concentrated to remove most of the THF, 300mL EA, 50mL water, 100mL EA aqueous phase extraction, combined organic phase, dried over anhydrous sodium sulfate, filtered, concentrated, column chromatography (PE: EA=5:1) 15.7 g of the product was obtained, and the yield was 99%.
[0124]
[0125] 1 H NMR(400MHz, CDCl 3 )δ6.83-7.45(m,13H), 5.16(s,2H), 4.66-4.69(m,1H), 4.26-4....
Embodiment 3
[0127] Step B
[0128] (2.23g, 5mmol) Compound 18 was added dropwise (2.0M, 3mL, 6mmol) NaHMDS in 50mL THF under the protection of argon at -78°C. After 1.5h at -78°C, (0.9mL, 6mmol) tert bromide was added dropwise Butyl ester, 2h TLC shows complete at this temperature, 10mL saturated ammonium chloride is dripped, 200mL EA extraction, 50mL saturated sodium chloride washing, anhydrous sodium sulfate drying, filtration, concentration, column chromatography (PE:EA=15:1 ) The product was 2.3 g, and the yield was 85%.
[0129]
[0130] 1 H NMR(400MHz, CDCl 3 )δ 6.79-7.43 (m, 13H), 5.42 (dd, J = 4.4 Hz, 11.2 Hz, 1H), 5.12 (s, 2H), 4.55-4.60 (m, 1H), 4.02-4.11 (m, 2H) ), 3.88(s,3H),3.24-3.39(m,2H), 2.78-2.81(m,1H), 2.60(dd,J=4.4Hz,16.8Hz,1H),1.43(s,9H); 13 C NMR(100MHz, CDCl 3 )δ173.5, 171.0, 152.8, 149.6, 147.7, 137.1, 135.7, 129.9, 129.5, 128.9, 128.6, 127.8, 127.3, 120.7, 113.9, 112.2, 80.9, 71.0, 65.7, 56.0, 55.8, 44.2, 40.2, 37.6, 28.1 ; HR-MS(ESI)calcd for C 32 H 35 O 7 NNa(M+Na) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com