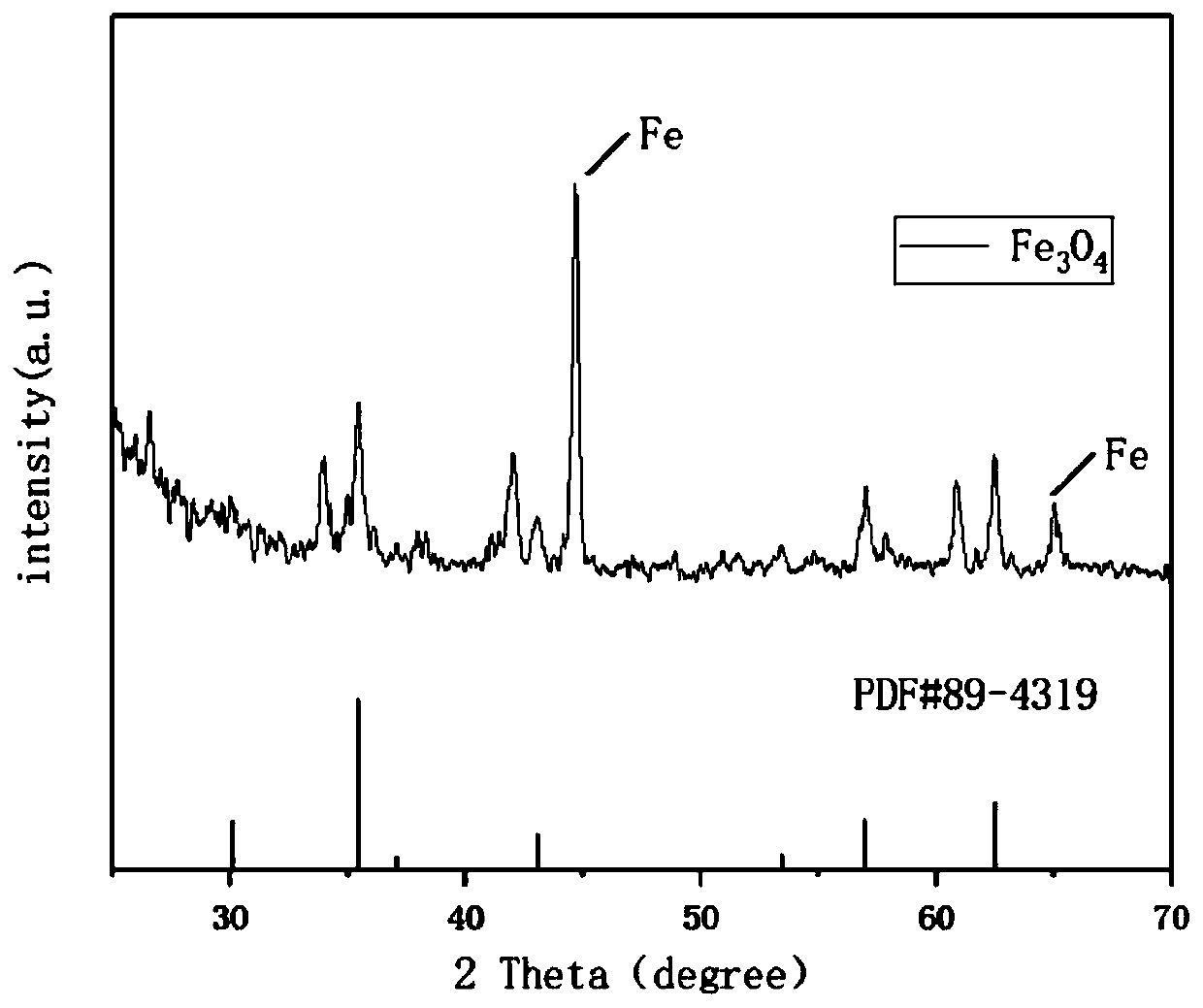
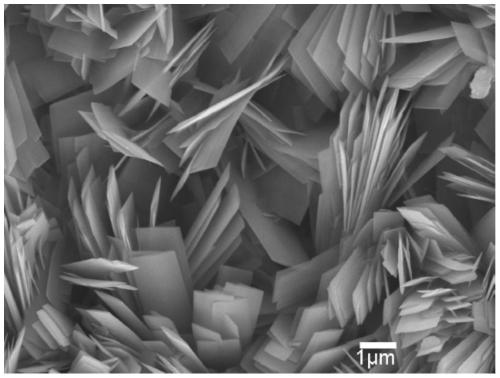
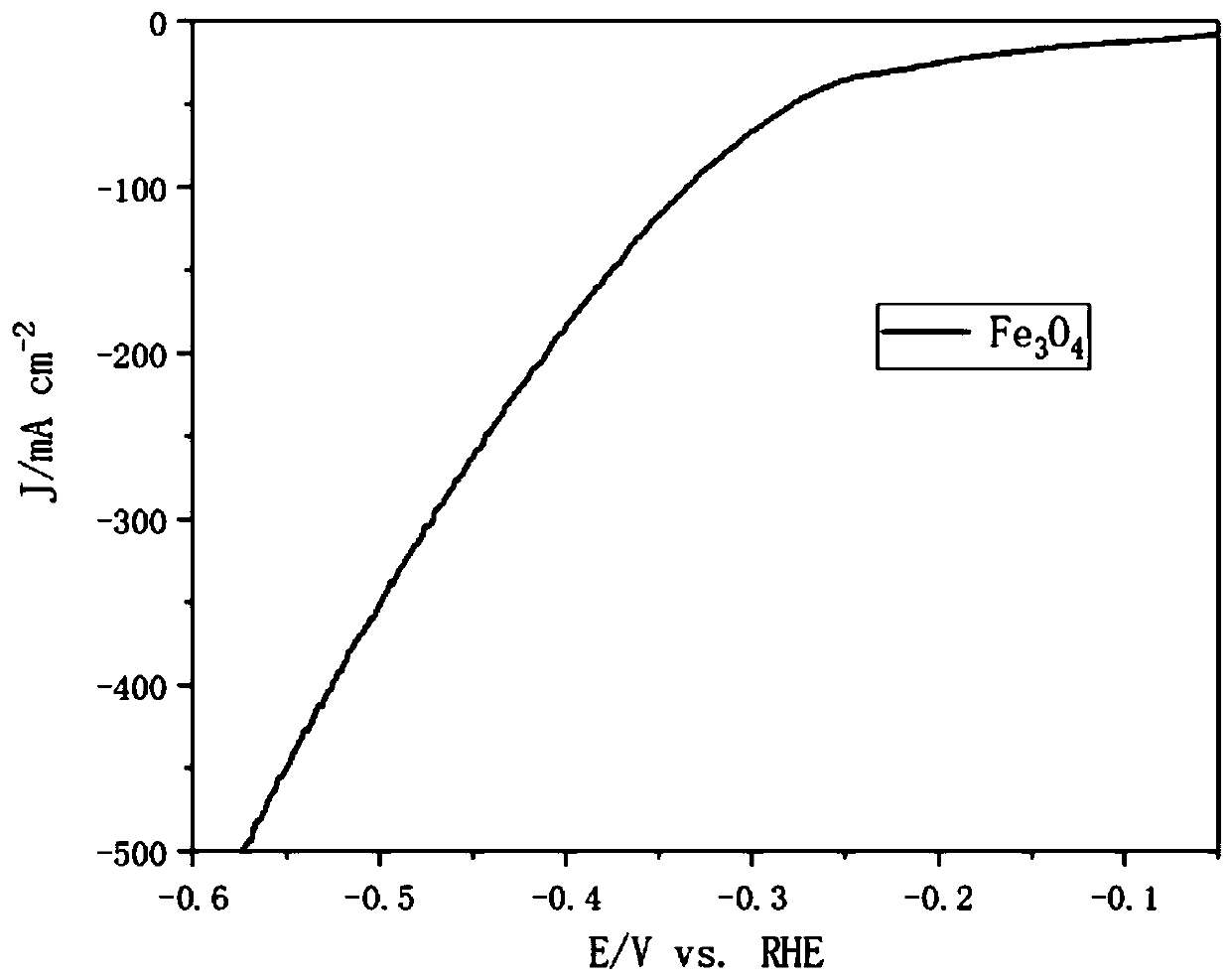
Iron oxide micron sheet self-supporting electrode and synthesis method thereof
A self-supporting electrode, iron oxide technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problems of complex preparation process, difficult to popularize in a large area, and high price of precious metals, to simplify experimental steps, save material consumption, and reduce electricity consumption. good chemical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] S1. Cut the foam iron to a size of 1×5cm; then clean the cut foam iron, first soak it in acetone for 10 minutes, then soak it in 3moL / L dilute hydrochloric acid for 20 seconds, and then wash it with ultrapure water and Rinse with ethanol three times alternately, and then put into deionized water for later use.
[0024] S2. Weigh 0.282 g of urea and dissolve it in 25 ml of ultrapure water, and stir to obtain a transparent and uniform urea aqueous solution.
[0025] S3, pour the stirred urea aqueous solution into a high-temperature hydrothermal kettle with a polytetrafluoroethylene liner, put the foam iron pretreated in step S1 into the polytetrafluoroethylene liner and seal it, and put it in an oven for reaction. The reaction time was 12 hours, and the temperature was 120°C.
[0026] S4. After the reaction is completed, the reaction kettle is cooled to room temperature, and the obtained product is cross-treated with ethanol and ultrapure water, and subjected to vacuum ...
Embodiment 2
[0028] S1. Cut the foam iron to a size of 1×5cm, and then clean the cut foam iron. First, soak it in acetone for 10 minutes, then soak it in 3moL / L dilute hydrochloric acid for 30 seconds, and then wash it with ultrapure water and Rinse with ethanol three times alternately, and then put into deionized water for later use.
[0029] S2. Weigh 0.47 g of urea and dissolve it in 25 ml of ultrapure water, and stir to obtain a transparent and uniform urea aqueous solution.
[0030] S3, pour the stirred urea aqueous solution into a high-temperature hydrothermal kettle with a polytetrafluoroethylene liner, put the foam iron pretreated in step S1 into the polytetrafluoroethylene liner and seal it, and put it in an oven for Reaction, the reaction time is 14 hours, and the temperature is 140°C.
[0031] S4. After the reaction is completed, the reaction kettle is cooled to room temperature, and the obtained product is cross-treated with ethanol and ultrapure water, and vacuum-dried. The v...
Embodiment 3
[0033] S1. Cut the foam iron to a size of 1×5cm, clean the cut foam iron, first soak it in acetone for 10 minutes, then soak it in 3moL / L dilute hydrochloric acid for 30 seconds, then wash it with ultrapure water and ethanol Rinse three times alternately, and then put it in ionized water for later use.
[0034] S2. Weigh 0.376 g of urea and dissolve it in 25 ml of ultrapure water, and stir to obtain a transparent and uniform urea aqueous solution.
[0035] S3, pour the stirred urea aqueous solution into a high-temperature hydrothermal kettle with a polytetrafluoroethylene liner, put the foam iron pretreated in step S1 into the polytetrafluoroethylene liner and seal it, and put it in an oven for reaction. The reaction time was 12 hours, and the temperature was 120°C.
[0036] S4. After the reaction is completed, the reaction kettle is cooled to room temperature, and the obtained product is cross-treated with ethanol and ultrapure water, and vacuum-dried. The vacuum-dried trea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com