Functional polypeptide and application
A technology with multiple functions and polypeptide fragments, applied in the field of medicine, can solve the problems of neglecting the complexity of AD pathogenesis, and achieve the effect of accelerating the clearance and reducing the content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
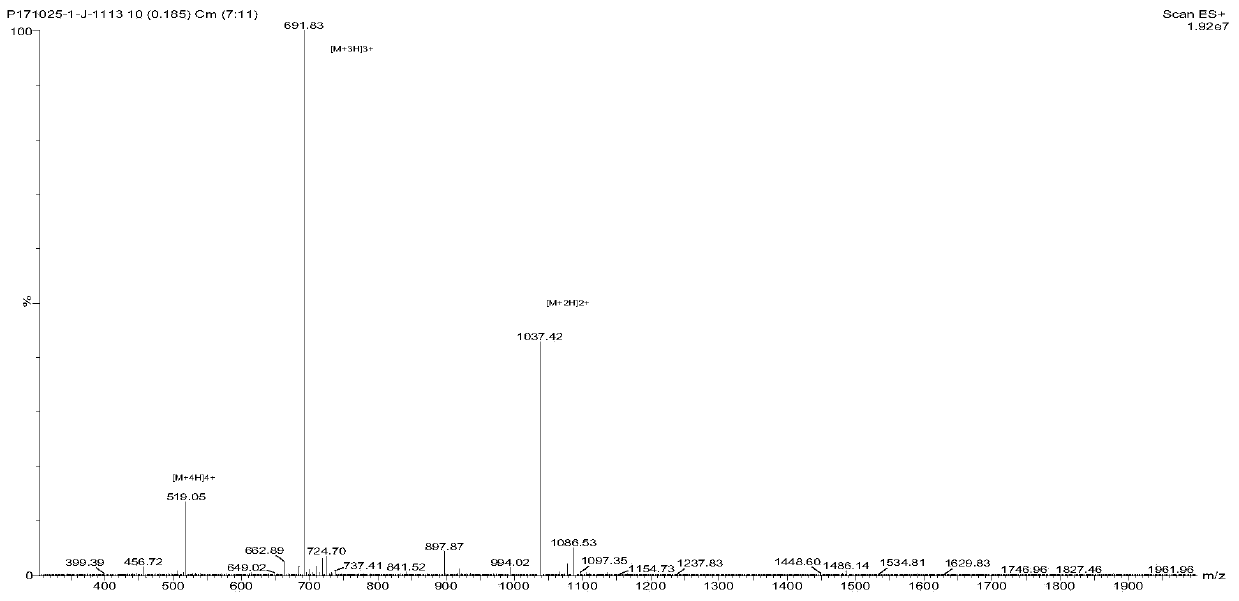
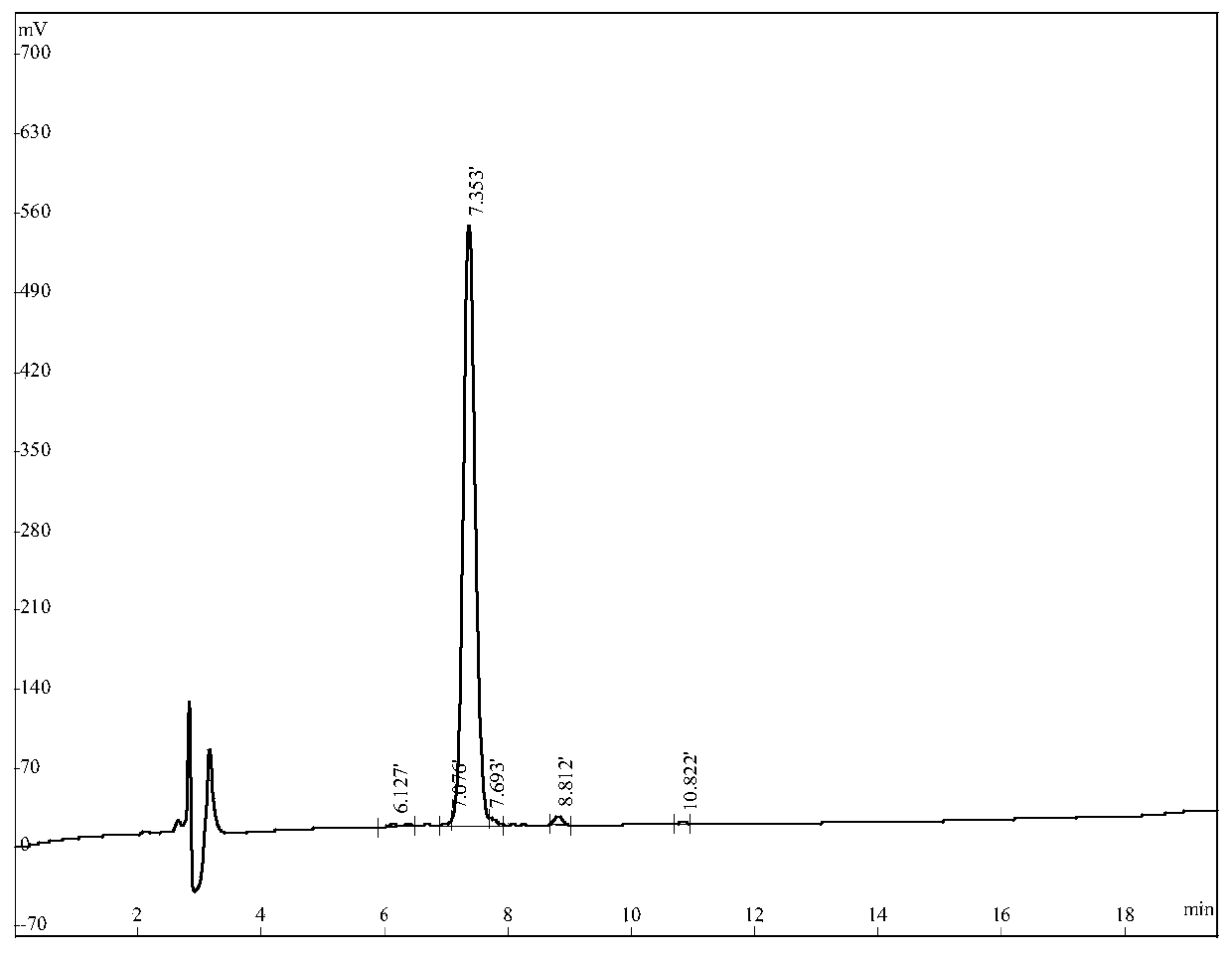
[0052] Embodiment 1: Preparation and purification of the functional polypeptide of the present invention
[0053] In this example, a solid-phase synthesis method was used to synthesize the multifunctional polypeptide MOP. Concrete synthetic steps are as follows:
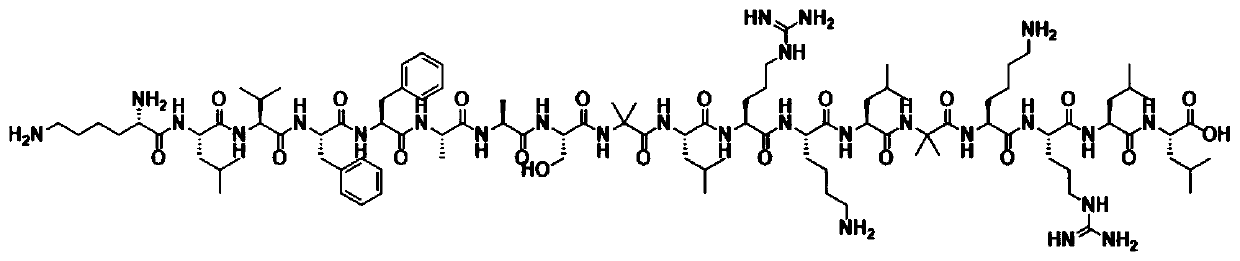
[0054] Using 9-fluorenylmethoxycarbonyl solid-phase peptide to synthesize said SEQ ID NO:16:Lys-Leu-Val-Phe-Phe-Ala-Ala-Ser-Aib-Leu-Arg-Lys-Leu-Aib-Lys -Arg-Leu-Leu
[0055] (KLVFFA-AS-Aib-LRKL-Aib-KRLL). These procedures were performed using a FocusXC automatic synthesizer (AAPPTEC). The MTT protecting group was removed from the KLVFFAAS-Aib-LRKL-Aib-KRLL sequence base (Rink-KLVFFA-AS-Aib-LRKL-Aib-KRLL-Fmoc), deprotected with TFA / TIS / DCM at 3:5:92 The mixture was shaken for 5 minutes to remove MTT, and the process was repeated twice. Convert the amino group at one end of leucine into carboxylic acid form, add succinic acid and DIEA to DMF to react with the polypeptide, and shake overnight at room temperature. ...
Embodiment 2
[0065] Example 2: The activity of the functional polypeptide of the present invention to inhibit the aggregation of Aβ
[0066] 1. ThT experiment of inhibiting Aβ monomer aggregation
[0067] The Aβ40 of 1mM stock concentration and the functional polypeptide of the present invention (fusion proteins shown in SEQ ID NO:16-22, respectively prepared) were diluted with phosphate buffered saline (PBS; 50mM, pH7.4, containing 150mM KCl), respectively A solution of 100 μM monomeric Aβ40 (mAβ40) and the functional polypeptide of the present invention was obtained. The fresh monomeric solution of Aβ40 (100 μM, 10 μL) was mixed with the functional polypeptide solution of the present invention (100 μM, 0, 1, 5, 10 μL), and the mixture was diluted with PBS to a final concentration of Aβ40 of 20 μM. The inhibitory activity of the functional polypeptide of the present invention on mAβ aggregation was determined in a black 96-well flat bottom plate. Subsequently, the plate was incubated at...
Embodiment 3
[0080] Example 3: MOP inhibits the induced cytotoxicity of Aβ aggregates:
[0081] The well-grown SH-SY5Y single cell suspension was mixed with 5×10 3 The density of cells / well was seeded in a 96-well plate with a volume of 200 μL per well. cells at 37°C, CO 2 After culturing at a concentration of 5% for 24 hours, the original medium was discarded, and the following samples were added to each well:
[0082] The mixture of Aβ40 (200 μM) and MOP incubated for 2 hours (Aβ:MOP=1:1, 1:0.5; 1:0.25), the stop concentration of Aβ40 is 2 μM; The cut-off concentration was 2 [mu]M; the A[beta]40 negative control contained everything except A[beta] and MOP (100% cell viability). The positive control group received 2% SDS solution (0% cell viability).
[0083] After continuing to culture the above 6 groups of cells for 48 hours, add 10 μL of MTT solution (5 mg / mL) to each well, incubate at 37 °C for 3 hours, terminate the culture, add 100 μL DMSO to each well to dissolve the crystals, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com