Acetolactate decarboxylase variants having improved specific activity
A technology of acetolactate decarboxylase and specific activity, applied in the direction of enzymes, lyases, carbon-carbon lyases, etc., can solve problems such as ALDC instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0201] The present disclosure is described in further detail in the following examples, which are not intended to limit the scope of the claimed disclosure in any way. The accompanying drawings are intended to be considered an integral part of the specification and description of this disclosure. The following examples are offered to illustrate but not limit the claimed disclosure.
example 1
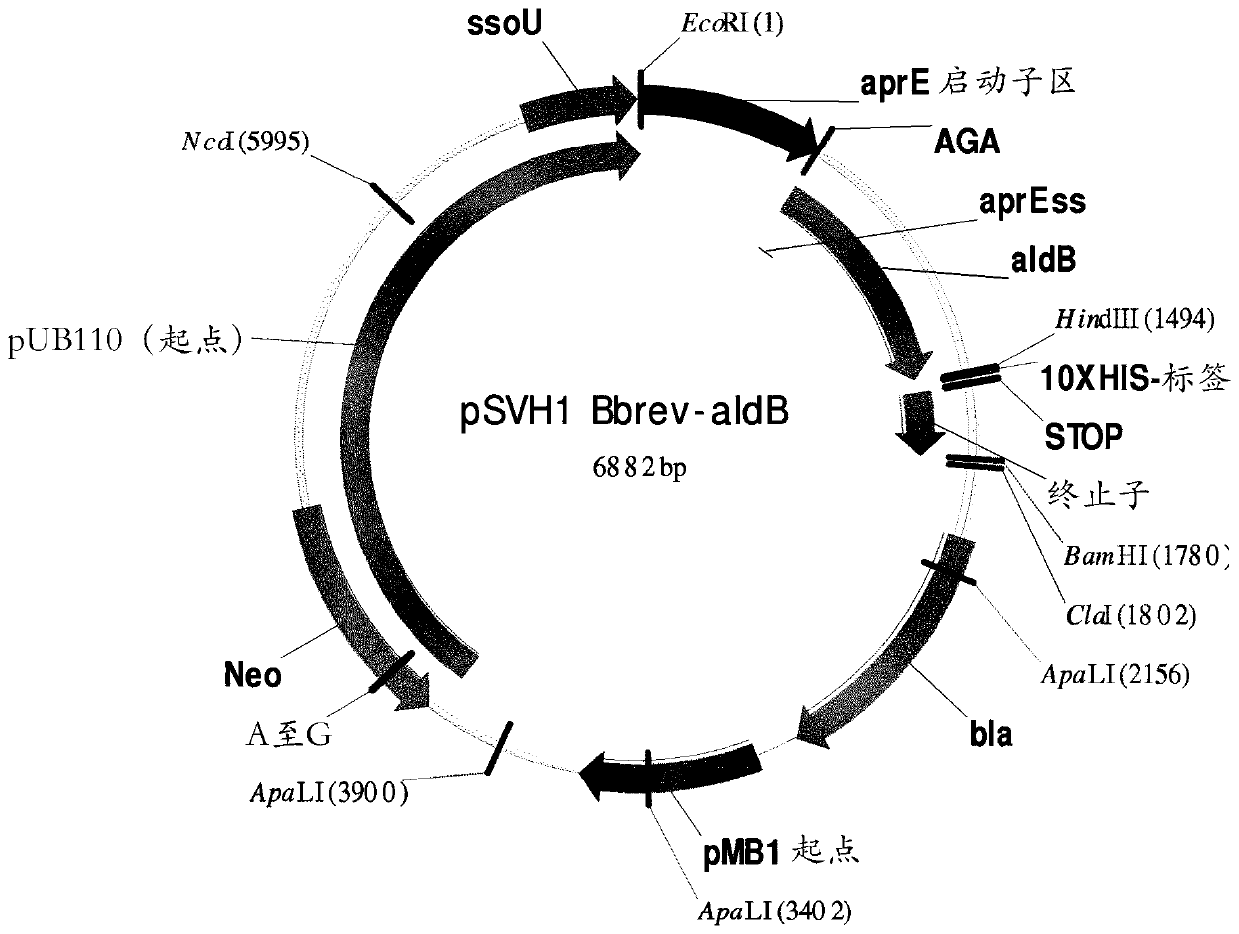
[0203] Heterologous expression of acetolactate decarboxylase ALDB
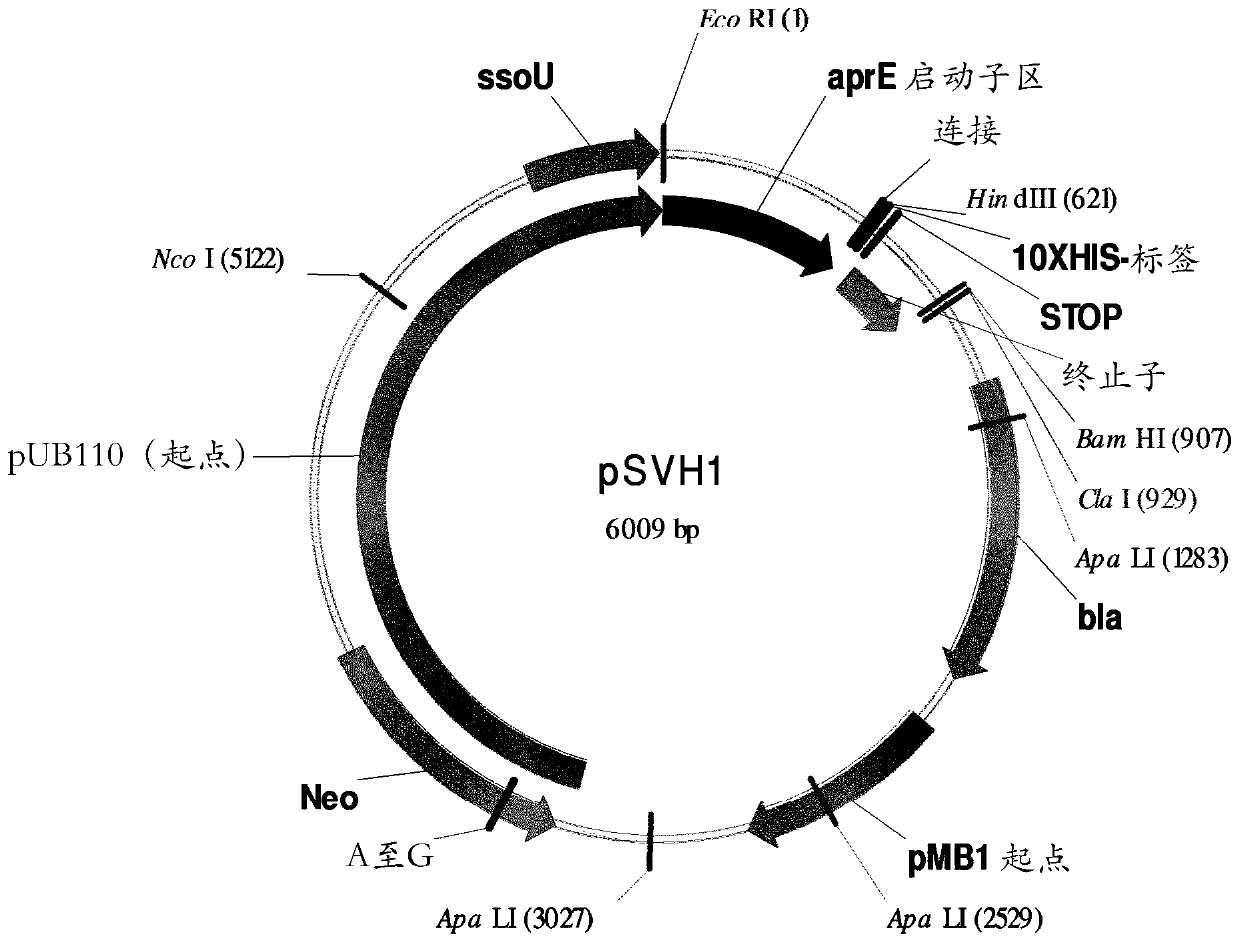
[0204] The Brevibacillus brevis (possibly called Bacillus brevis) acetolactate decarboxylase (ALDC) aldB gene was previously identified (Diderichsen et al., J Bacteriol. (1990) 172(8): 4315), whose sequence is shown in UNIPROT accession number P23616.1. The sequence number of this gene, aldB, is described in SEQ ID NO:1. Nucleotides highlighted in bold and underlined are those encoding the signal peptide. The aldB gene and the corresponding encoded zymogen are also referred to as wild type (WT).
[0205] SEO ID NO: 1 lists the nucleotide sequence of the aldB gene:
[0206]
[0207] The zymogen encoded by the aldB gene is described in SEQ ID NO:2. At the N-terminus, the protein has a signal peptide of 24 amino acids in length as predicted by SignalP-NN (Emanuelsson et al., Nature Protocols (2007) 2:953-971). The signal peptide sequence is underlined and shown in bold in SEQ ID NO:2. The presence of a s...
example 2
[0237] Protein Assay by Stain Free Imager Criterion
[0238] Using Gel Doc TM The EZ Imaging System quantifies proteins by SDS-PAGE gels and densitometry. Reagents used in the assay: Concentrated (2x) Laemmli sample buffer (Bio-Rad, catalog #161-0737); 26-well XT4-12% Bis-Tris gel (Bio-Rad , catalog #345-0125); protein markers "Precision Plus Protein Standards (Precision Plus Protein Standards)" (Bio-Rad, catalog #161-0363); protein standards BSA (Thermo Scientific , catalog #23208) and SimplyBlue Safestain (Invitrogen, catalog #LC 6060). The assay was performed as follows: 50 μL of the diluted enzyme sample was mixed with 50 μL of sample buffer containing 2.7 mg DTT in a 96-well PCR plate. The plate was sealed with Microseal 'B' membrane from Bio-Rad and placed in a PCR machine heated to 70°C for 10 minutes. Afterwards, fill the chamber with running buffer and set up the gel cassette. Then 10 μL of each sample and standard (0.125-1.00 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com