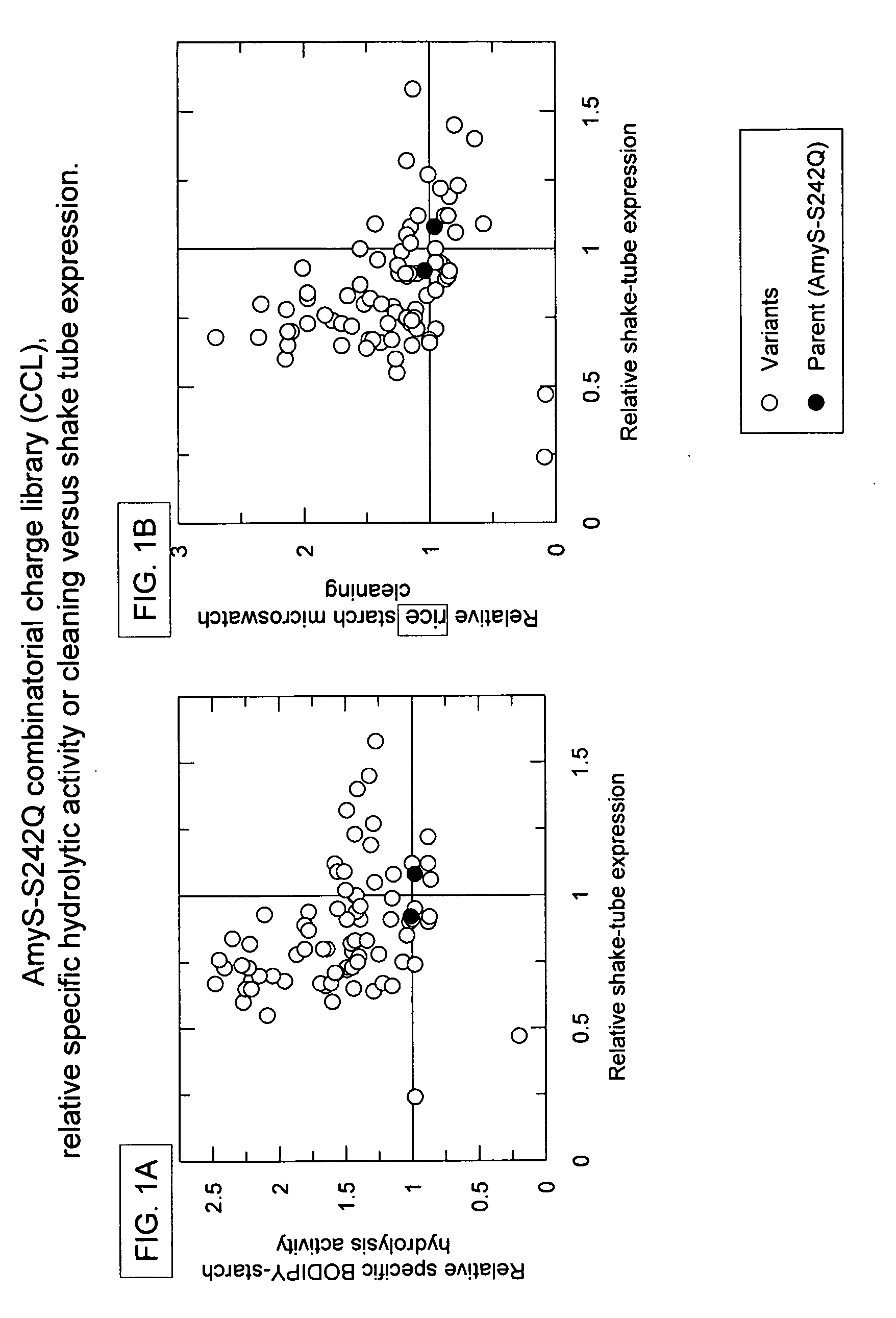
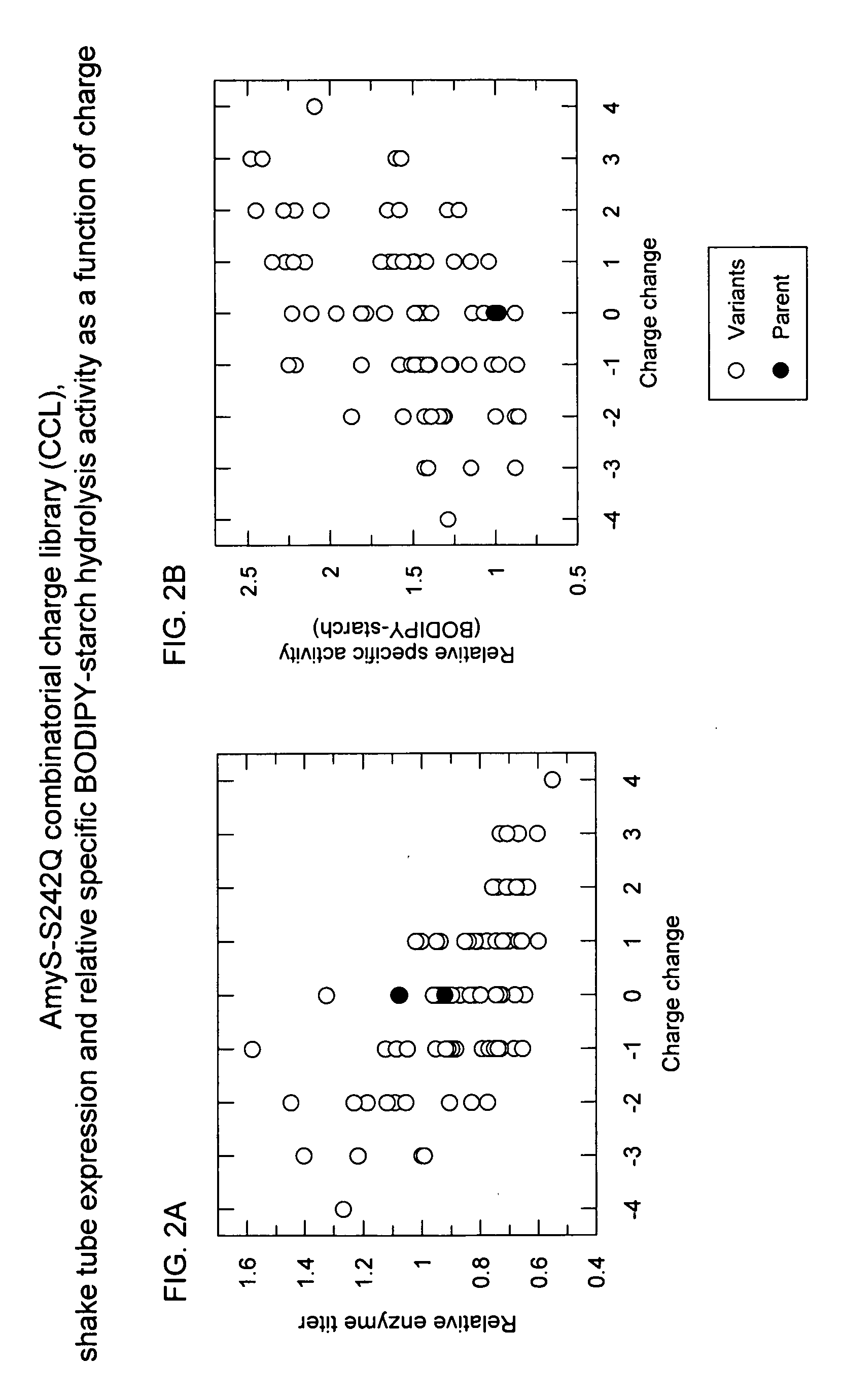
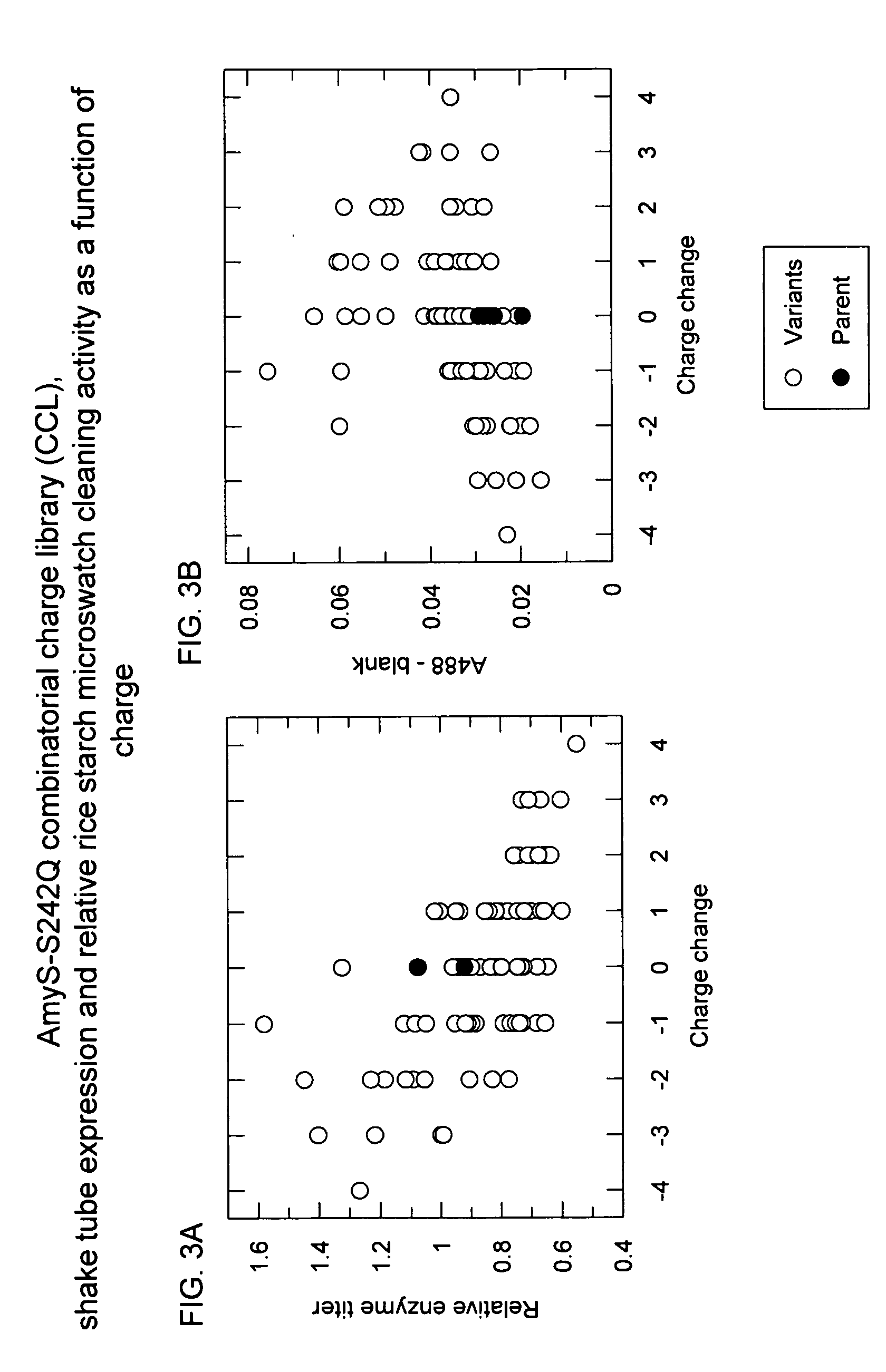
Methods for Improving Protein Properties
a technology of protein properties and methods, applied in the field of protein engineering, can solve problems such as inapplicability of conclusions, and achieve the effects of optimizing catalytic activity and/or stability, improving performance and/or stability, and optimizing storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assays
[0173]The following assays were used in the examples described below. Any deviations from the protocols provided below are indicated in the examples. In these experiments, a spectrophotometer was used to measure the absorbance of the products formed after the completion of the reactions. A reflectometer was used to measure the reflectance of the swatches.
A. Protein Content Determination
[0174]1. BCA (bicinchoninic acid) Assay for Protein Content Determination
[0175]In these assays, BCA (Pierce) assay was used to determine the protein concentration in protease samples on a microtiter plate (MTP) scale. In this assay system, the chemical and reagent solutions used were: BCA protein assay reagent, and Pierce Dilution buffer (50 mM MES, pH 6.5, 2 mM CaCl2, 0.005% TWEEN®-80). The equipment used was a SpectraMAX (type 340) MTP reader. The MTPs were obtained from Costar (type 9017).
[0176]In the test, 200 μl BCA reagent was pipetted into each well, followed by 20 μl diluted protein. Aft...
example 2
NprE Protease Production in B. subtilis
[0216]In this Example, experiments conducted to produce NprE protease in B. subtilis are described. In particular, the methods used in the transformation of plasmid pUBnprE into B. subtilis are provided. Transformation was performed as known in the art (See e.g., WO 02 / 14490, and U.S. patent application Ser. No. 11 / 581,102). The DNA sequence (nprE leader, nprE pro and nprE mature DNA sequence from B. amyloliquefaciens) provided below encodes the NprE precursor protein.
(SEQ ID NO: 1)GTGGGTTTAGGTAAGAAATTGTCTGTTGCTGTCGCCGCTTCCTTTATGAGTTTAACCATCAGTCTGCCGGGTGTTCAGGCCGCTGAGAATCCTCAGCTTACAAAGTGGAGCATGCCGCCACAACCGGAACAGGTACGACTCTTAAAGGAAAAACGGTCTCATTAAATATTTCTTCTGAAAGCGGCAAATATGTGCTGCGCGATCTTTCTAAACCTACCGGAACACAAATTATTACGTACGATCTGCAAAACCGCGAGTATAACCTGCCGGGCACACTCGTATCCAGCACCACAAACCAGTTTACAACTTCTTCTCAGCGCGCTGCCGTTGATGCGCATTACAACCTCGGCAAAGTGTATGATTATTTCTATCAGAAGTTTAATCGCAACAGCTACGACAATAAAGGCGGCAAGATCGTATCCTCCGTTCATTACGGCAGCAGATACAATAACGCAGCCTGGATCGGCGAC...
example 3
Generation of Site Evaluation Libraries (SELs)
[0224]In this Example, methods used in the construction of nprE SELs are described.
[0225]The pUBnprE vector, containing the nprE expression cassette described above, served as template DNA. This vector contains a unique BglII restriction site, which was utilized in the site evaluation library construction. Briefly, to construct a nprE site evaluation library, three PCR reactions were performed, including two mutagenesis PCRs to introduce the mutated codon of interest in the mature nprE DNA sequence and a third PCR used to fuse the two mutagenesis PCRs in order to construct the pUBnprE expression vector including the desired mutated codon in the mature nprE sequence.
[0226]The method of mutagenesis was based on the codon-specific mutation approach, in which the creation of all possible mutations at a time in a specific DNA triplet was performed using a forward and reverse oligonucleotide primer with a length of 25 to 45 nucleotides enclosi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com