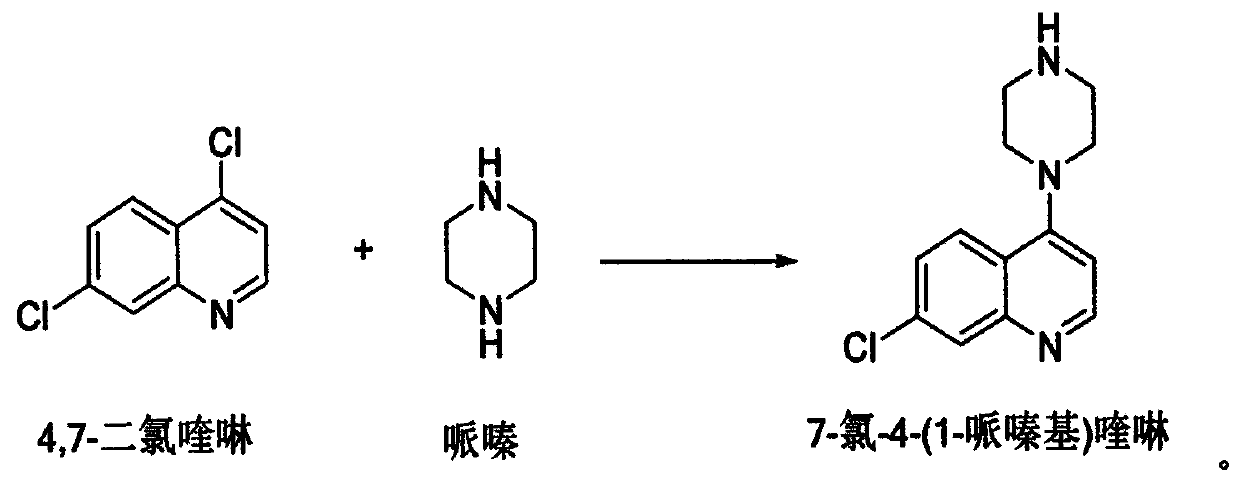
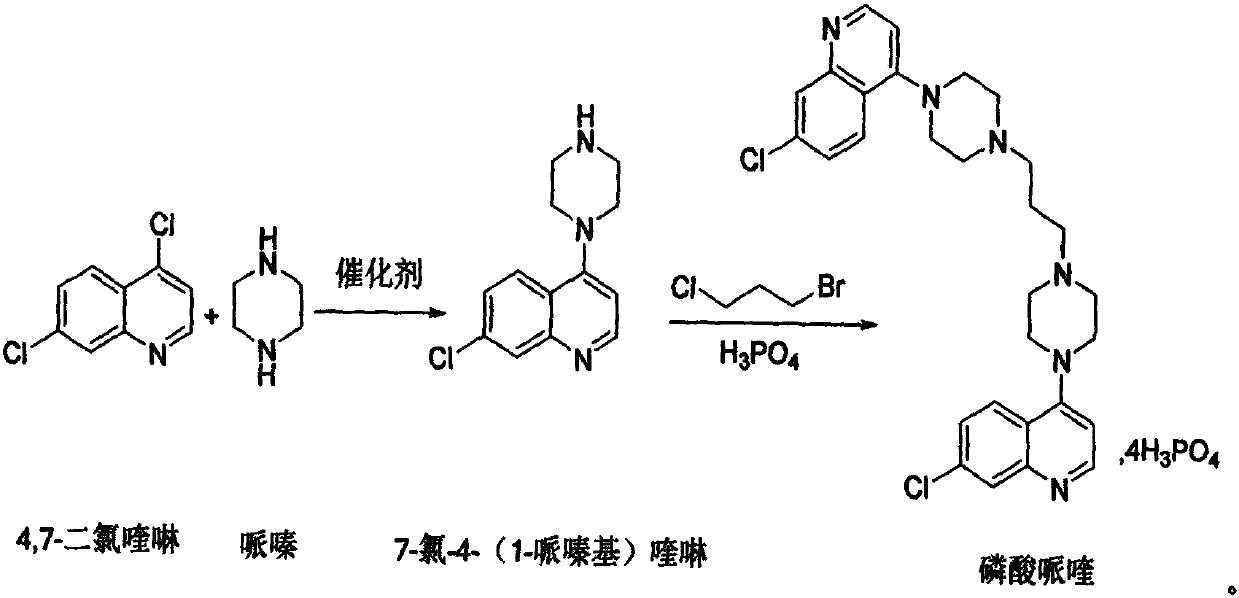
Method for synthesizing piperaquine intermediate in continuous flow microreactor
An intermediate, piperaquine technology, applied in the field of synthesis of intermediate 7-chloro-4-quinoline, can solve the problems of low yield and poor purity, and achieve high yield, short processing flow and few by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment provides a method for utilizing a microchannel reactor to synthesize piperaquine phosphate key intermediate 7-chloro-4-(1-piperazinyl)quinoline, and the synthetic method is as follows:
[0029] (1) take by weighing 198g4, the dichloromethane that 7-dichloroquinoline adds 594g, after stirring, fully stir and mix to dissolving clear formation material I, material I is delivered to the preheating module of microchannel reactor and carries out preheating;
[0030] (2) Take by weighing 430g piperazine and add 215g of drinking water, fully mix to form material II after stirring, and material II is delivered to the preheating module of microchannel reactor for preheating;
[0031] (3) The preheated material I and material II carry out condensation reaction in the reaction module group, wherein: the flow rate of the slurry pump is adjusted so that the flow rate of the material 1 is 30.0g / min, and the flow rate of the slurry pump is adjusted so that the material I...
Embodiment 2
[0033] (1) take by weighing 198g4, the toluene that 7-dichloroquinoline adds 802g, after stirring, fully stir and mix to dissolve clear and form material I, material I is delivered to the preheating module of microchannel reactor and carry out preheating;
[0034] (2) Take by weighing 430g piperazine and add 215g of drinking water, fully mix to form material II after stirring, and material II is delivered to the preheating module of microchannel reactor for preheating;
[0035](3) The preheated material I and material II carry out condensation reaction in the reaction module group, wherein: the flow velocity of the adjustment slurry pump makes the flow velocity of the material I be 38.0g / min, and the flow velocity of the adjustment slurry pump makes the material I The flow rate of the flow rate is 54.5g / min, the condensation reaction temperature is 109 ℃, the mol ratio of dichloroquinoline and piperazine is 1: 5, and the total residence time of reaction in the reaction module g...
Embodiment 3~9
[0036] Embodiment 3~9: Based on embodiment 1, change the temperature of reaction, other conditions are unchanged, the result that obtains is as follows table.
[0037]
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com