Application of glutathione (GSH) to preparation of drug for preventing and treating central nervous system infection caused by salmonella typhimurium
A Salmonella, central nervous system technology, applied in the field of medicine, can solve problems such as intestinal flora disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Application of GSH in the treatment of CNS infection caused by Salmonella typhimurium
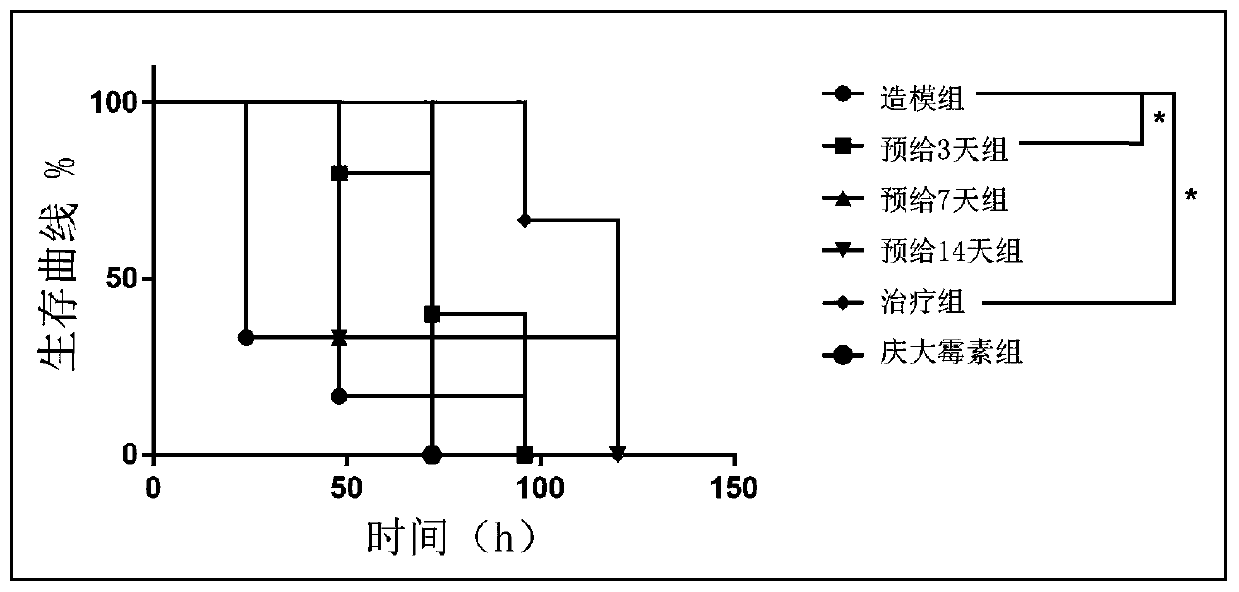
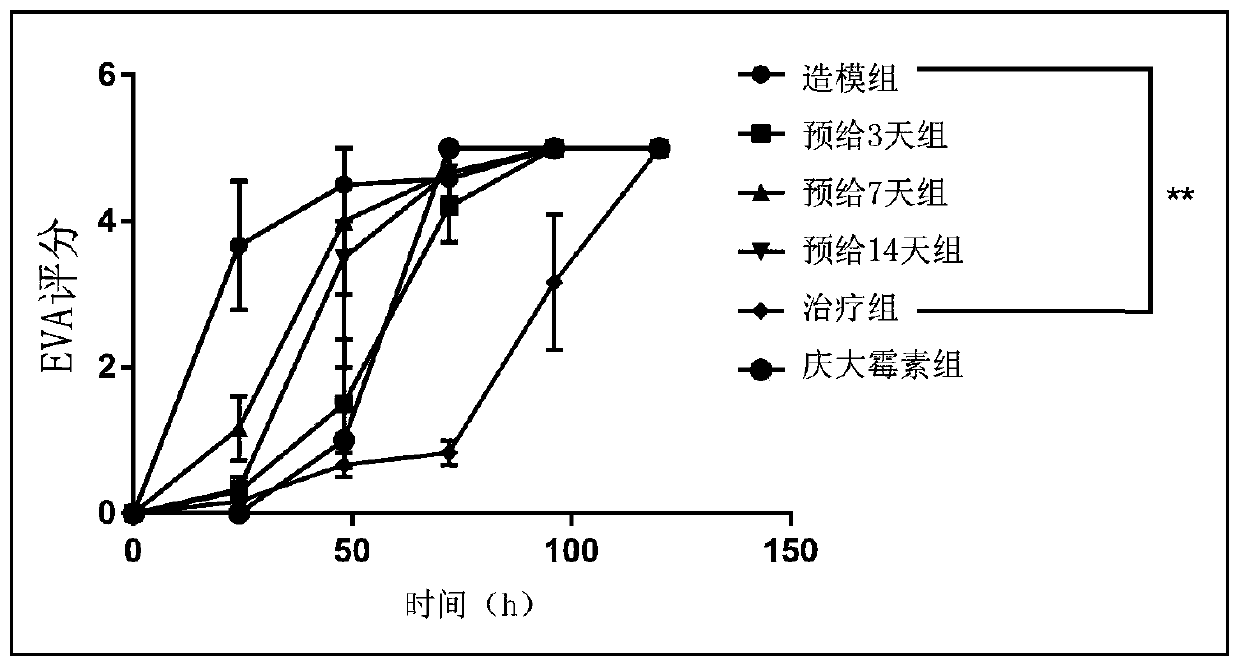
[0013] Step 1: Experimental grouping (8-week-old C57 female mice, n=6)
[0014] i) Modeling group: pre-administered equal volume of normal saline (0.2mL / 20g) for 14 days. After infection, an equal volume of normal saline (0.2mL / 20g) was administered intragastrically.
[0015] ii) GSH pre-administration group for three days: after 11 days pre-administration of equal volume of normal saline (0.2 mL / 20 g), pre-administration of GSH (500 mg / kg*day) for 3 days. After infection, an equal volume of normal saline (0.2mL / 20g) was administered intragastrically.
[0016] iii) Seven-day GSH pre-administration group: after 7-day pre-administration of equal volume of normal saline (0.2 mL / 20 g), 7-day pre-administration of GSH (500 mg / kg*day). After infection, an equal volume of normal saline (0.2mL / 20g) was administered intragastrically.
[0017] iv) Fourteen-day GSH pre-administration group:...
Embodiment 2
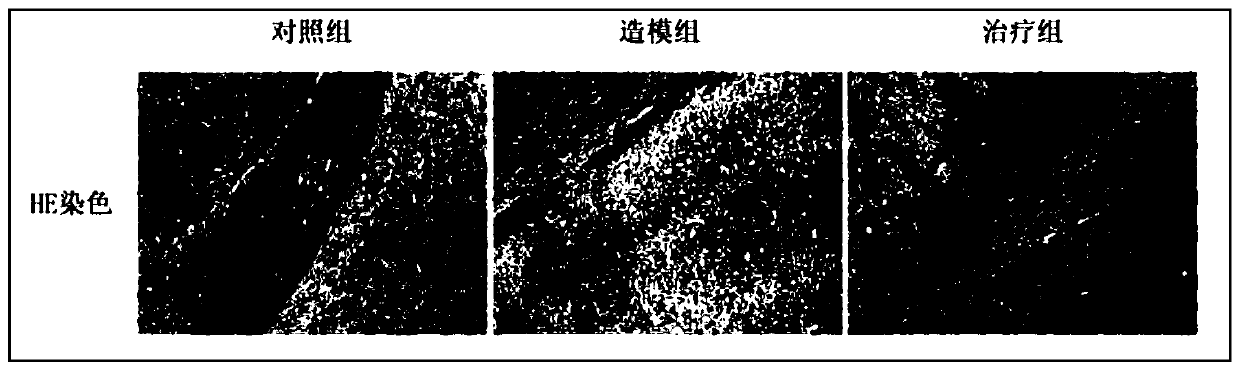
[0027] Pharmacological Study of GSH in Treating CNS Infection Caused by Salmonella Typhimurium
[0028] Step 1: Experimental grouping (8-week-old C57 female mice, n=6)
[0029] i) Control group: an equal volume of normal saline (0.2 μL) was injected into the ventricle of the brain, and an equal volume of normal saline (0.2 mL / 20 g) was intragastrically administered after suturing.
[0030] ii) Modeling group: Salmonella typhimurium (65,000 CFU) was injected unilaterally into the ventricle, and an equal volume of normal saline (0.2 mL / 20 g) was intragastrically administered after suturing.
[0031] iii) Treatment group: Salmonella typhimurium (65000 CFU) was injected unilaterally into the ventricle, and an equal volume of GSH (500 mg / kg) was intragastrically administered after suturing.
[0032] Step 2: Establish a CNS infection model
[0033] i) Cultivation of Salmonella typhimurium SL1344: When cultivating the bacterial liquid, pick a single colony with a sterilized white g...
Embodiment 3
[0054] Study on the Mechanism of GSH Alleviating Cerebral Apoptosis
[0055] Step 1: Experimental grouping (8-week-old C57 female mice, n=6)
[0056] i) Control group: an equal volume of normal saline (0.2 μL) was injected into the ventricle of the brain, and an equal volume of normal saline (0.2 mL / 20 g) was intragastrically administered after suturing.
[0057] ii) Modeling group: Salmonella typhimurium (65,000 CFU) was injected unilaterally into the ventricle, and an equal volume of normal saline (0.2 mL / 20 g) was intragastrically administered after suturing.
[0058] iii) Treatment group: Salmonella typhimurium (65000 CFU) was injected unilaterally into the ventricle, and an equal volume of GSH (500 mg / kg) was intragastrically administered after suturing.
[0059] Step 2: Establish a CNS infection model
[0060] i) Culture of Salmonella typhimurium SL1344: When cultivating the bacterial liquid, pick a single colony with a sterilized white gun tip, pour into LB broth liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com