Preparing method of acridine
A technology of acridine and carboxyl, which is applied in the field of preparation of acridine, can solve the problems of low yield, achieve high purity and yield, good industrial application value, and reduce the effect of industrial manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method embodiment 1 of acridine of the present invention, synthetic route is as follows:
[0019]
[0020] Specifically adopt the following steps to prepare:
[0021] Add 9-carboxyacridine 3.13kg (14.03mol) in the reactor with thermometer and reflux device, cuprous oxide 0.02kg (0.14mol), o-phenanthroline 0.02kg (0.11mol), NMP (N-methyl pyrrolidone) 4.17kg (42.09mol), reacted at 140°C for 8h, evaporated the NMP solvent after the reaction, added 1.5L of water and mixed evenly, filtered to obtain a black crude product, and recrystallized the black crude product with petroleum ether to obtain acridine The light yellow solid was 2.35kg, the purity was 99.1%, and the yield was 93.0%.
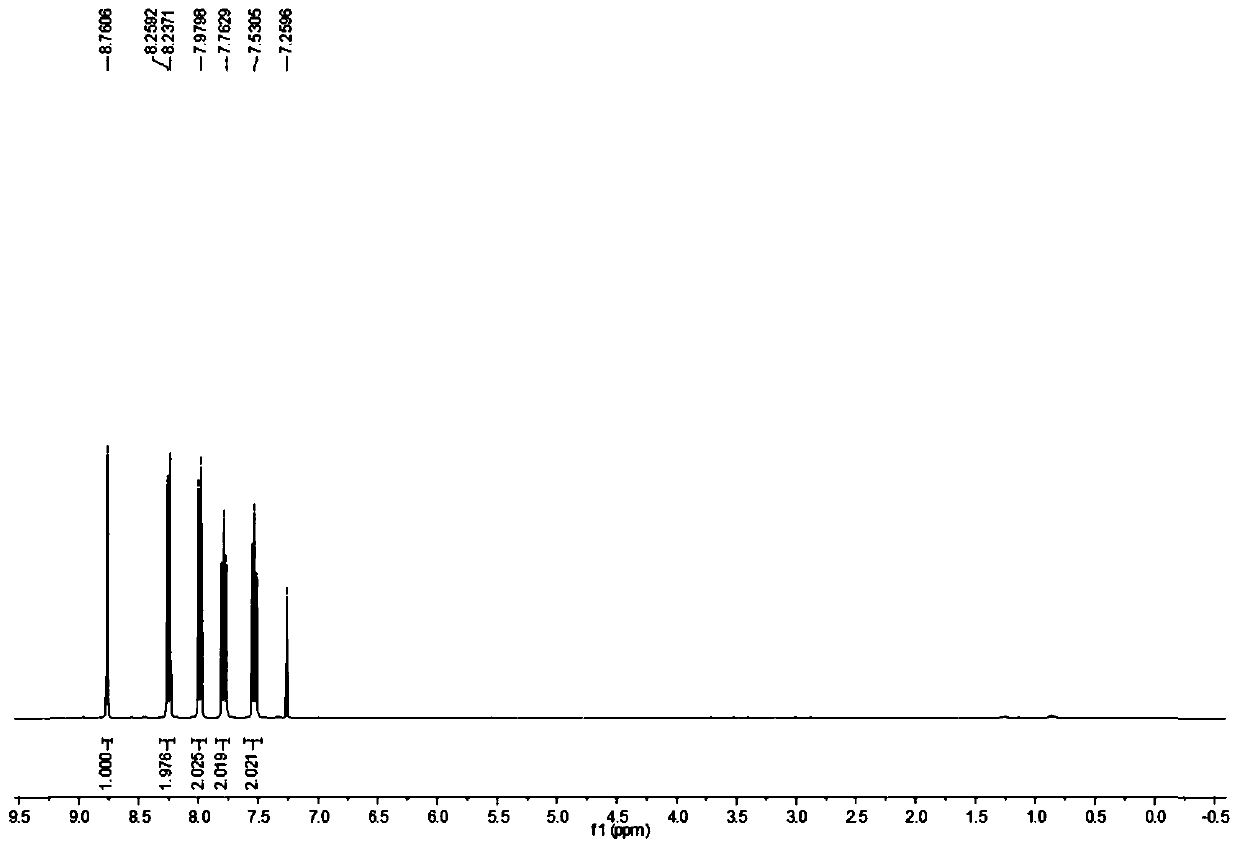
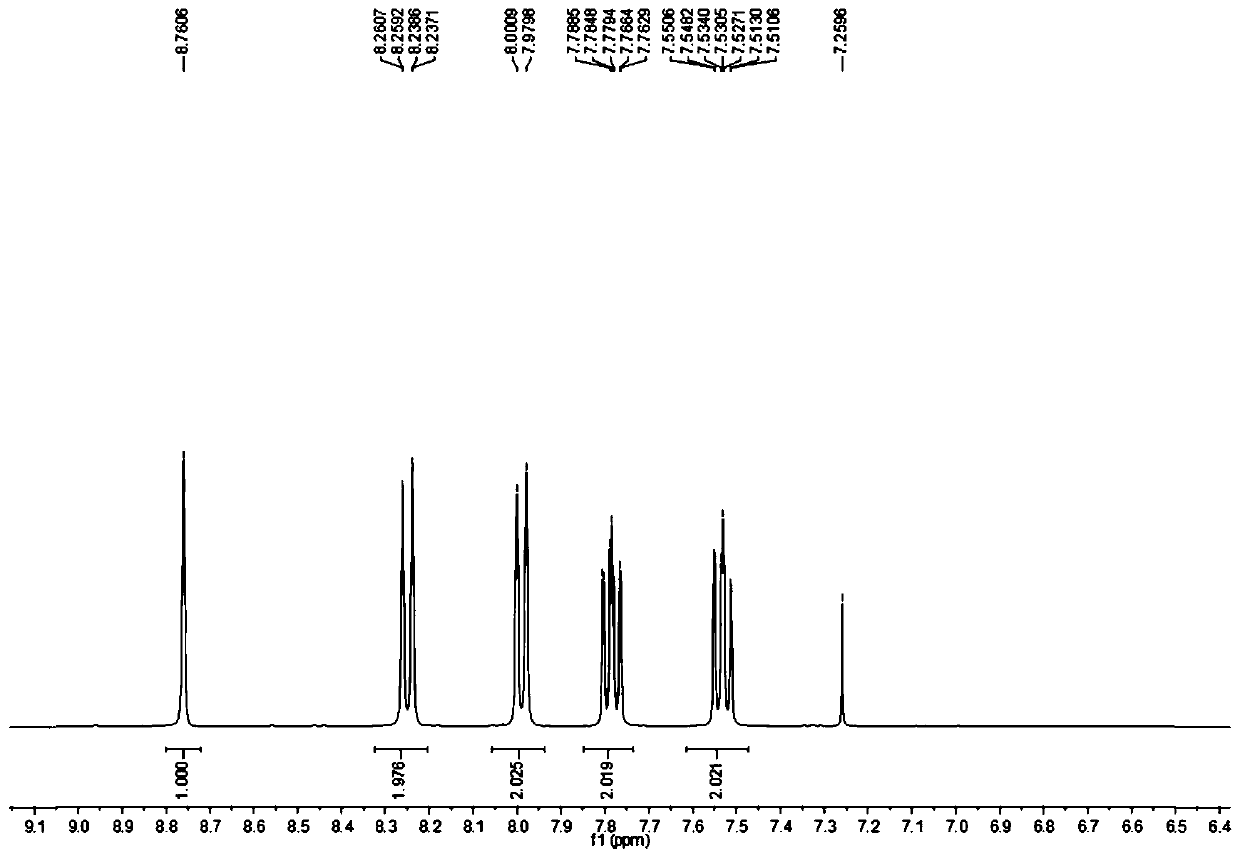
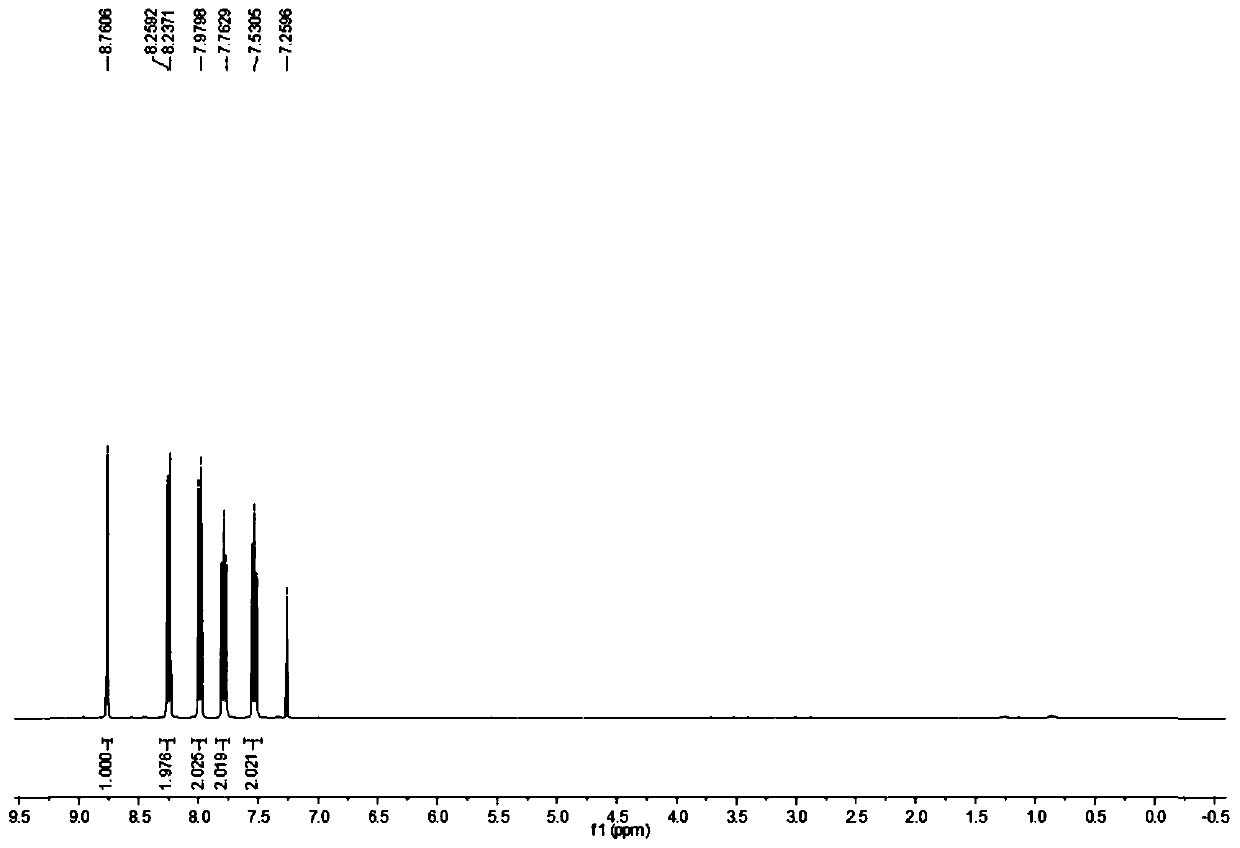
[0022] The NMR H spectrum of the product is as figure 1 and figure 2 Shown, the characteristic data are: HNMR (CDCl 3 , 400Hz) δ: 8.76 (s, 1H); 8.24-8.26, (d, 2H, J=8); 7.98-8.00 (d, 2H, J=8); 7.76-7.79 (m, 2H); 7.51- 7.55 (m, 2H).
Embodiment 2
[0023] The preparation method embodiment 2 of acridine of the present invention, synthetic route is as follows:
[0024]
[0025] Specifically adopt the following steps to prepare:
[0026] Add 9-carboxyacridine 1.16kg (5.20mol) in the reactor with thermometer and reflux device, cuprous iodide 0.001kg (0.005mol), o-phenanthroline 0.001kg (0.005mol), NMP 2.56kg ( 25.86mol), reacted at 170°C for 6h, evaporated the NMP solvent after the reaction, added 0.56L of water and mixed evenly, filtered to obtain a black crude product, and recrystallized the black crude product with cyclohexane to obtain 0.086kg of acridine as a light yellow solid , with a purity of 99.5% and a yield of 91.7%. The NMR characterization data of the product are consistent with Example 1.
[0027] The preparation method embodiment 3 of acridine of the present invention, synthetic route is as follows:
[0028]
[0029] Specifically adopt the following steps to prepare:
[0030] Add 1.25kg (5.60mol) of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com