New synthetic process of anticancer drug CF-102
A technology of CF-102 and anticancer drugs, which is applied in the new synthesis process field of anticancer drug CF-102, which can solve the problems of uneconomical reaction routes, high production costs, cumbersome operations, etc., and achieve low production costs and low equipment requirements , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention.
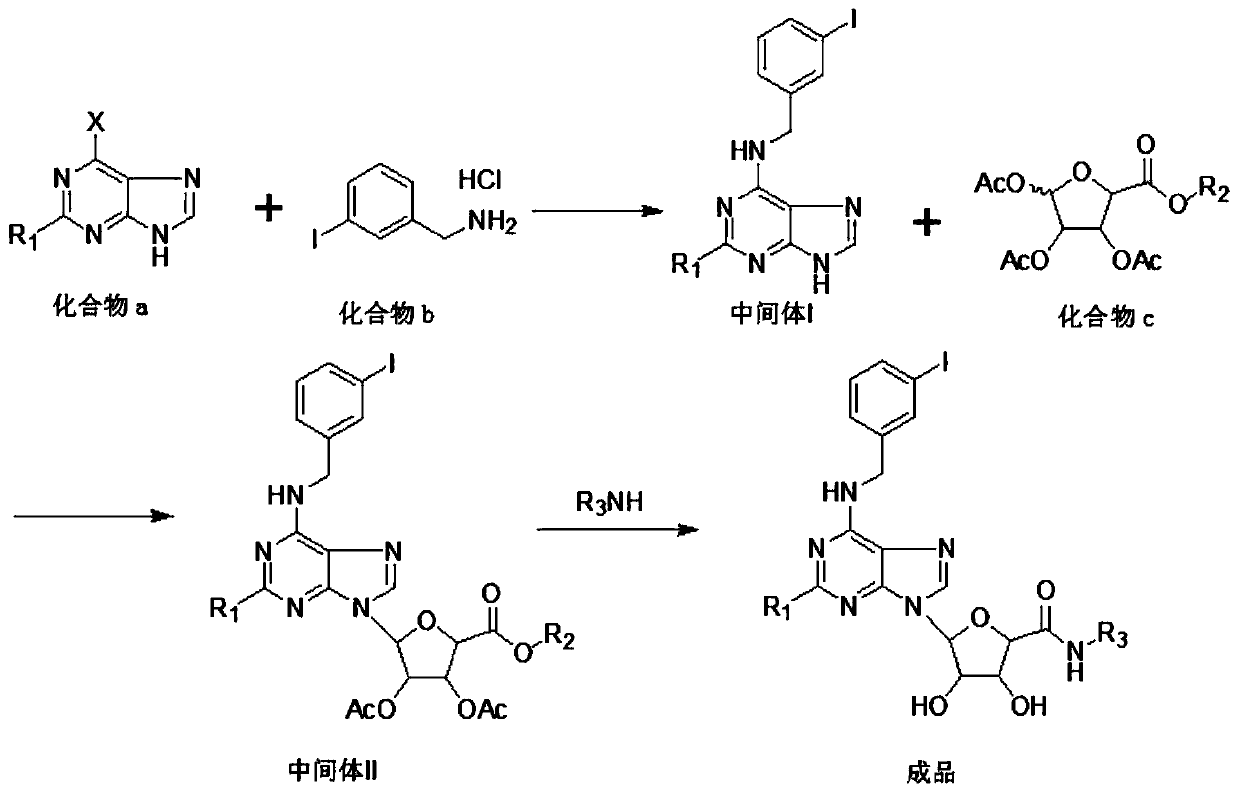
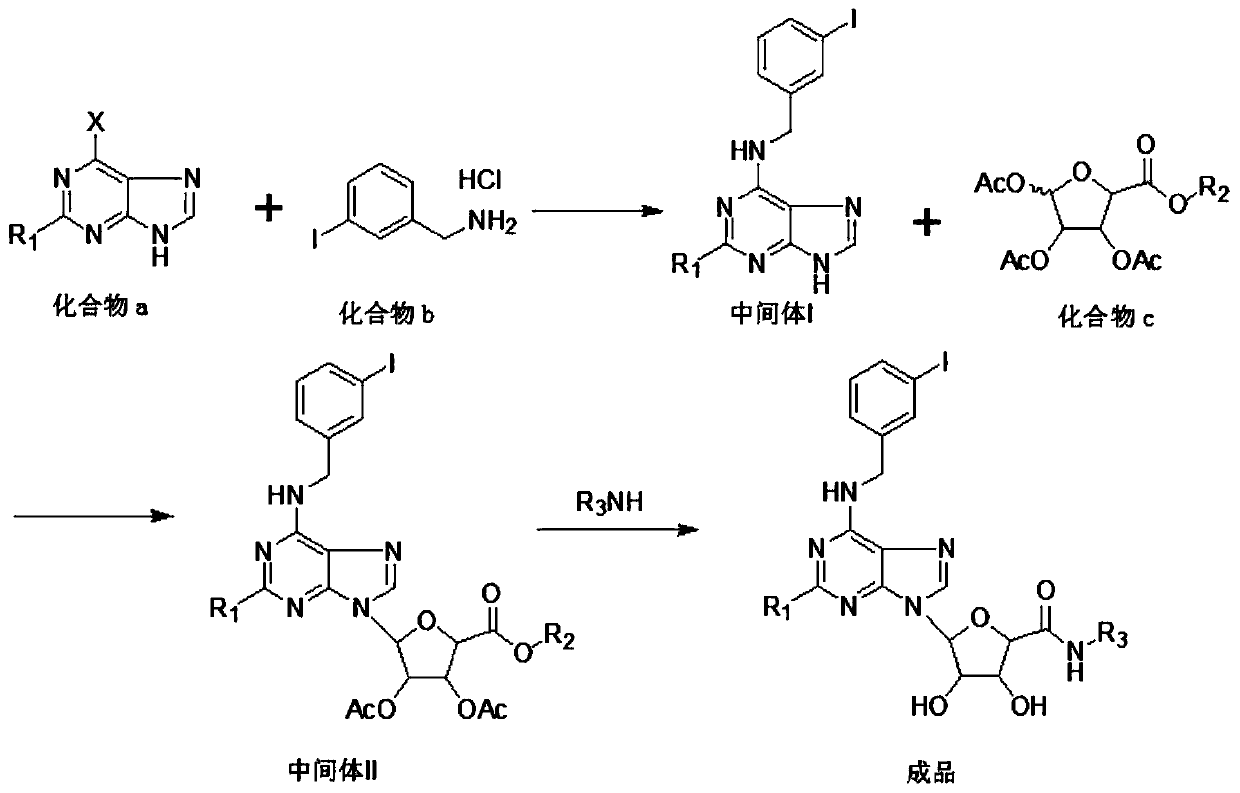
[0017] see figure 1 , an embodiment provided by the present invention: a new synthesis process of anticancer drug CF-102, comprising the following steps:
[0018] Step 1: add compound a: 189g (1.0mol) 2,6-dichloropurine and 303g triethylamine (3mol) in 3000ml dry, clean there-necked flask, add 323.4g m-iodobenzylamine hydrochloride and 1200ml acetonitrile , Stir and heat up to produce substitution reaction, to reflux for 24h; Substitution reaction occurs, cool down to 0-5°C, crystallize for 2h, obtain light yellow solid weight, dry under reduced pressure at 60°C to constant weight, solid dry weight: 329.7g, mol Yield 85.5%, to obtain intermediate I;
[0019] Step 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com