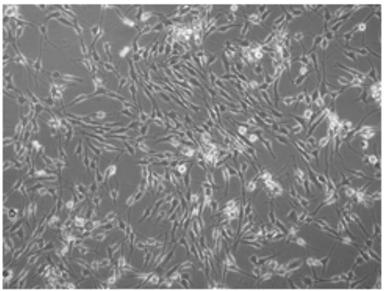
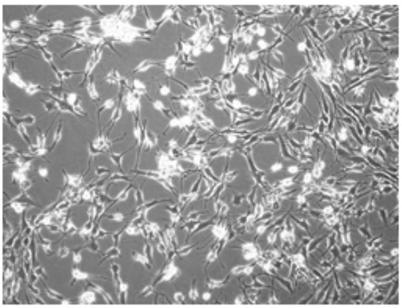
Method for in vitro proliferation of chondrocytes
A technology for chondrocytes and in vitro expansion, applied in the direction of bone/connective tissue cells, animal cells, vertebrate cells, etc., can solve the problems of ineffective application, selection of collection sites and limited collection volume, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0087] According to a preferred embodiment of the present invention, the extract of Rhizoma Rhizoma Rhizoma and Rhizoma Drynariae extract are obtained as follows:
[0088] Step a, take 1 to 5 g, for example 2 g, of Rhizoma Rhizoma and Rhizoma Rhizoma Drynaria respectively, and then wash them with triple distilled water;
[0089] Step b. Soak in 200-800mL triple-distilled water, such as 400mL triple-distilled water, for 0.2-2h, such as 1h, and then simmer for 0.1-1h, such as 0.5h;
[0090] Step c, filtering respectively to obtain the Rhizoma Rhizoma Extract and Rhizoma Rhizoma Drynaria Extract.
[0091] According to a preferred embodiment of the present invention, in step 3, three-dimensional cell culture is used for subculture of cells.
[0092] In a further preferred embodiment, in step 3, the cells are subcultured using the polymer as a three-dimensional scaffold.
[0093] In a further preferred embodiment, in step 3, the cells are subcultured with polyε-caprolactone-b-pol...
Embodiment 1
[0113] Chopped cartilage into 1.0mm 3 Size, washed 3 times with Ham's F12 medium (containing 10mmol / L HEPES buffer, 70μmol / L gentamicin sulfate, 22μmol / L amphotericin B, 300μmol / L ascorbic acid);
[0114] Put it into 9 mL of digestion medium (containing 1.5 mg / mL type II collagenase prepared in PBS) for digestion at 37 ° C for 15 hours, and then filter through a nylon mesh with a diameter of 40 μm;
[0115] Centrifuge at a speed of 1400 rpm, collect the cell pellet, and obtain chondrocytes;
[0116] 2500 pieces / cm 2 The cell density was seeded at 50cm 2 In the cell culture bottle, let stand for 2 days, wherein, the cell culture bottle is placed in the cell culture box (37 ℃, 5.0% CO 2 ), according to the GMP international quality control technical standard, the chondrocytes are placed in air cleanliness of 10000 grades, under the condition of 100 grades of biological safety cabinets for expansion and culture;
[0117] Carry out in DMEM medium (containing 8% human serum, 30...
Embodiment 2
[0121] Chopped cartilage into 1.2mm 3 Size, washed 3 times with Ham's F12 medium (containing 15mmol / L HEPES buffer, 60μmol / L gentamicin sulfate, 25μmol / L amphotericin B, 250μmol / L ascorbic acid);
[0122] Put it into 10mL digestion medium (containing 2mg / mL type II collagenase prepared in PBS) and digest at 37°C for 13 hours, then filter through a nylon mesh with a diameter of 40μm;
[0123] Carry out centrifugation at a speed of 1300 rpm, collect cell pellets, and obtain chondrocytes;
[0124] 3500 pieces / cm 2 The cell density was seeded at 50cm 2 In the cell culture bottle, let stand for 2.5 days, wherein, the cell culture bottle is placed in the cell culture box (37 ℃, 5.0% CO 2 ), according to the GMP international quality control technical standard, the chondrocytes are placed in air cleanliness of 10000 grades, under the condition of 100 grades of biological safety cabinets for expansion and culture;
[0125] Cells were cultured in DMEM medium (containing 10% human s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com