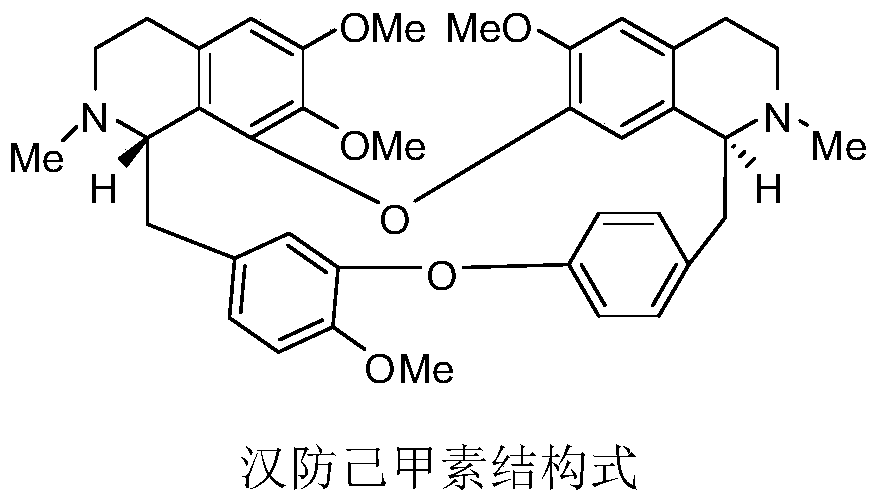
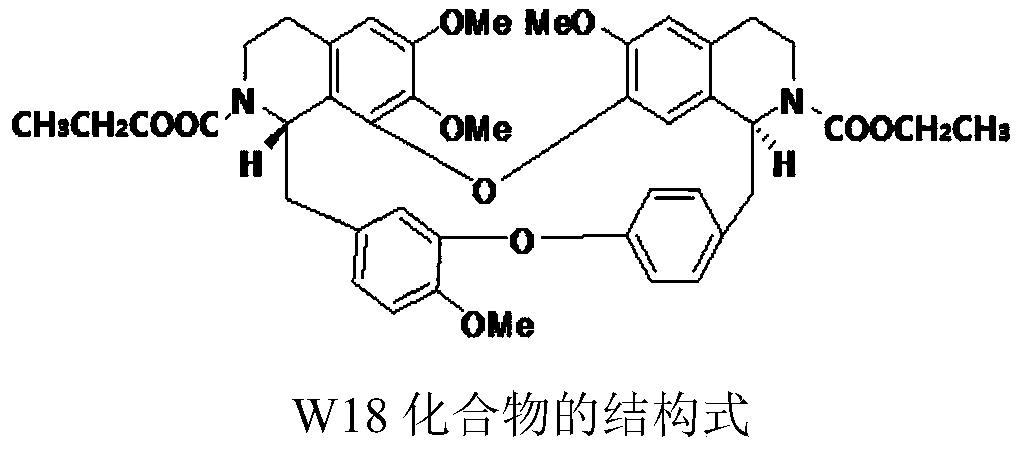
Medical composition of didemethyl tetrandrine dual ethyl formate and tyrosine kinase inhibitor
A technology of ethyl bisformate and tyrosine kinase, which is applied in the field of medicine and can solve problems such as treatment failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synergistic sensitization effect of W18 combined with sorafenib on cytotoxicity of intrinsically drug-resistant hepatocellular carcinoma Bel7402
[0027] Bel7402 cells in the logarithmic growth phase were digested with trypsin, mixed evenly, and seeded in a 96-well culture plate at a seeding concentration of 1200 cells / well. After culturing for 24 hours, add Sorafenib (1.0 μM, 10 μM, 25 μM), or W18 (0.25 μM, 0.5 μM, 1.0 μM, 3.0 μM), or a combination of the two drugs, and set 3 parallel wells for each drug concentration . After continuing to cultivate for 72 hours, discard the culture medium, add 0.5 mg / ml MTT 100 μl (dissolved in serum-free RPMI1640 culture solution) to each well, continue to cultivate for 4 hours, discard MTT, add 150 μl of DMSO to each well, shake with a mixing shaker, and use a microplate reader Measure the absorbance value at the wavelength of 570nm, and calculate the cytotoxicity (inhibition rate) with the following formula: inhibition rate (%)=(1...
Embodiment 2
[0032] Synergistic sensitization effect of W18 combined with regorafenib on cytotoxicity of acquired drug-resistant hepatocellular carcinoma HepG2 / sor
[0033] HepG2 / sor cells in the logarithmic growth phase were digested with trypsin, mixed evenly, and inoculated in a 96-well culture plate at a seeding concentration of 2000 cells / well. After culturing for 24 hours, add regorafenib (1.0 μM, 10 μM, 25 μM), or W18 (0.25 μM, 0.5 μM, 1.0 μM, 3.0 μM), or a combination of the two drugs, and set 3 parallel wells for each drug concentration . After continuing to cultivate for 72 hours, discard the culture medium, add 0.5 mg / ml MTT 100 μl (dissolved in serum-free RPMI1640 culture solution) to each well, continue to cultivate for 4 hours, discard MTT, add 150 μl of DMSO to each well, shake with a mixing shaker, and use a microplate reader Measure the absorbance value at the wavelength of 570nm, and calculate the cytotoxicity (inhibition rate) with the following formula: inhibition rate...
Embodiment 3
[0039] Synergistic inhibitory effect of W18 combined with sorafenib on the proliferation of Bel7402 single cells
[0040] For Bel7402 cells in exponential growth phase, make cell suspension. Cells were counted, and the cell concentration was adjusted with medium, ready for use. Dilute the cell suspension several times, and inoculate 5ml of the cell suspension into culture dishes (diameter 60mm) according to the concentration of 200 cells per dish, and gently shake the culture dish in a cross direction to make the cells evenly dispersed. Place the Petri dish at 37°C, 5% CO 2 After culturing for 24 hours, the cells adhered to the wall, added Sorafenib (1.0 μM, 10 μM, 25 μM), or W18 (0.25 μM, 0.5 μM, 1.0 μM, 3.0 μM), or a combination of the two drugs, and cultured for another 2 to 3 weeks. When clones were visible to the naked eye in the culture dish, the culture was terminated, the culture medium was discarded, carefully soaked twice in PBS solution, and air-dried. Fix with m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com