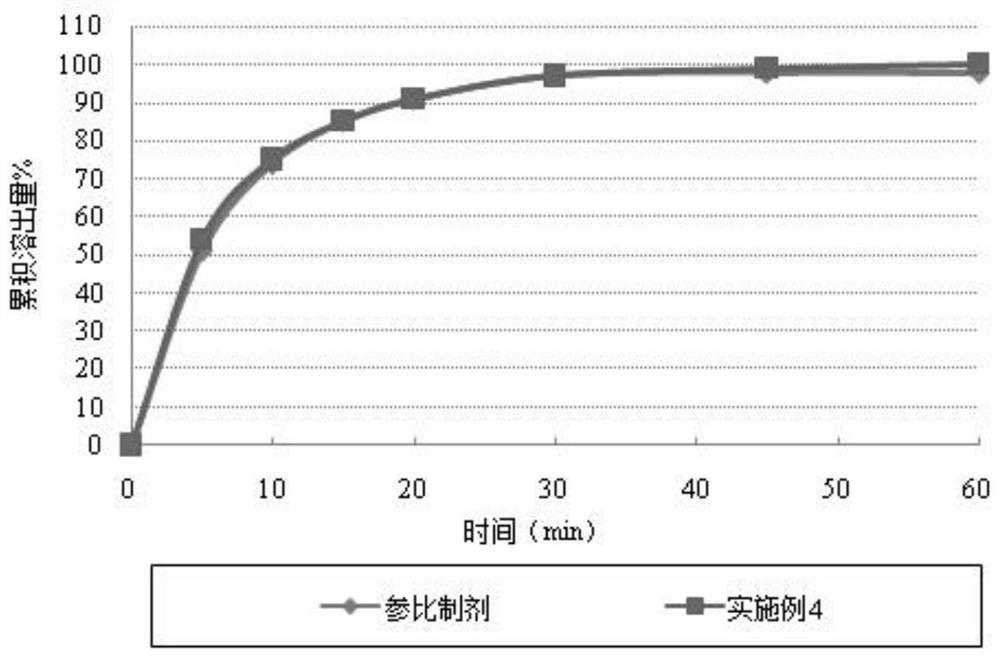
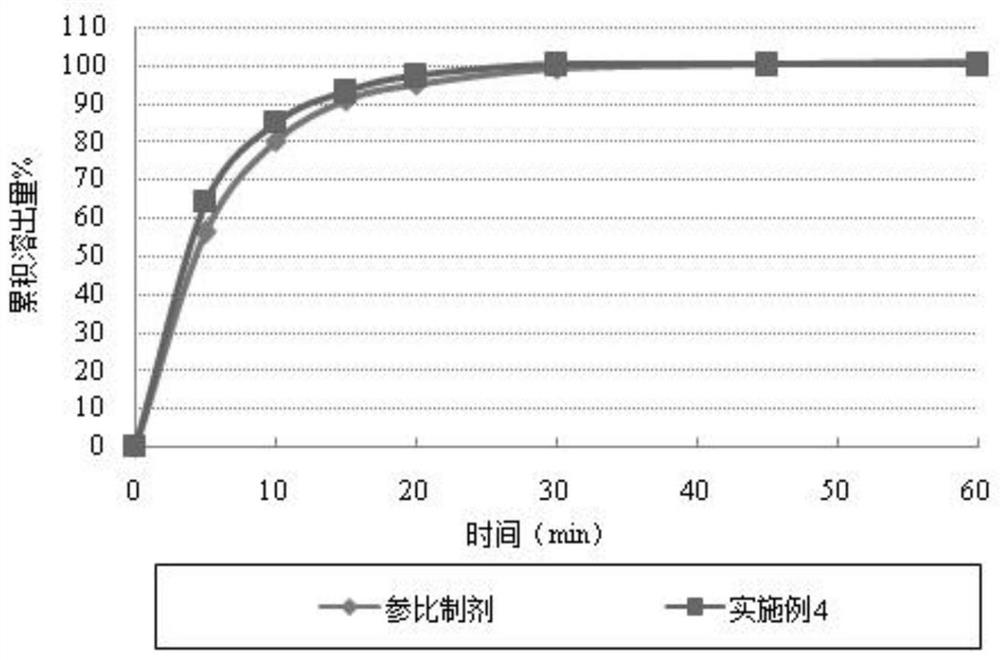
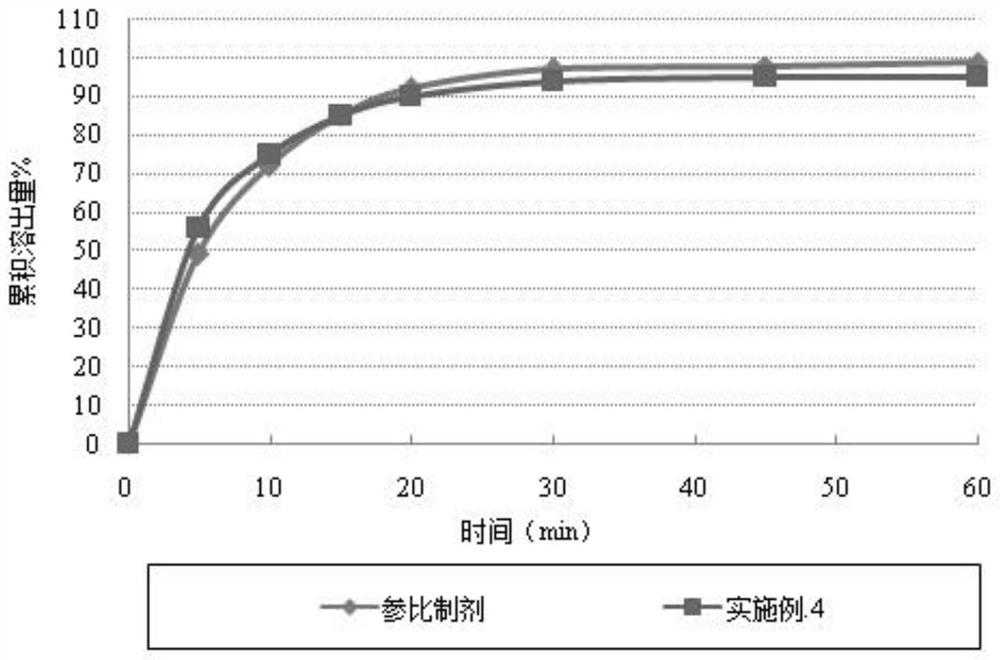
A kind of cefuroxime axetil solid preparation and preparation technology thereof
A technology for cefuroxime axetil and solid preparation, which can be applied in the directions of pill delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as great harm and safety disputes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of cefuroxime axetil solid preparation, the present embodiment is tablet, and tablet core formula is:
[0046]
[0047] The preparation process of the above-mentioned cefuroxime axetil tablet is the same as that of the comparative example, and glyceryl behenate is used instead of hydrogenated castor oil, and a tablet machine is used to compress it into cefuroxime axetil tablets each weighing 450 mg.
Embodiment 2
[0049]A kind of cefuroxime axetil solid preparation, the present embodiment is tablet, and tablet core formula is:
[0050]
[0051] The preparation process of the above-mentioned cefuroxime axetil tablet is the same as that of the comparative example, using sodium stearyl fumarate instead of hydrogenated castor oil, and using a tablet machine to compress into cefuroxime axetil tablets each weighing 450 mg.
Embodiment 3
[0053] A kind of cefuroxime axetil solid preparation, the present embodiment is tablet, and tablet core formula is:
[0054]
[0055] The preparation process of the above-mentioned cefuroxime axetil tablet is the same as that of the comparative example, the hydrogenated castor oil is replaced by talcum powder, and the cefuroxime axetil tablet with a weight of 450 mg is compressed by a tablet machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com