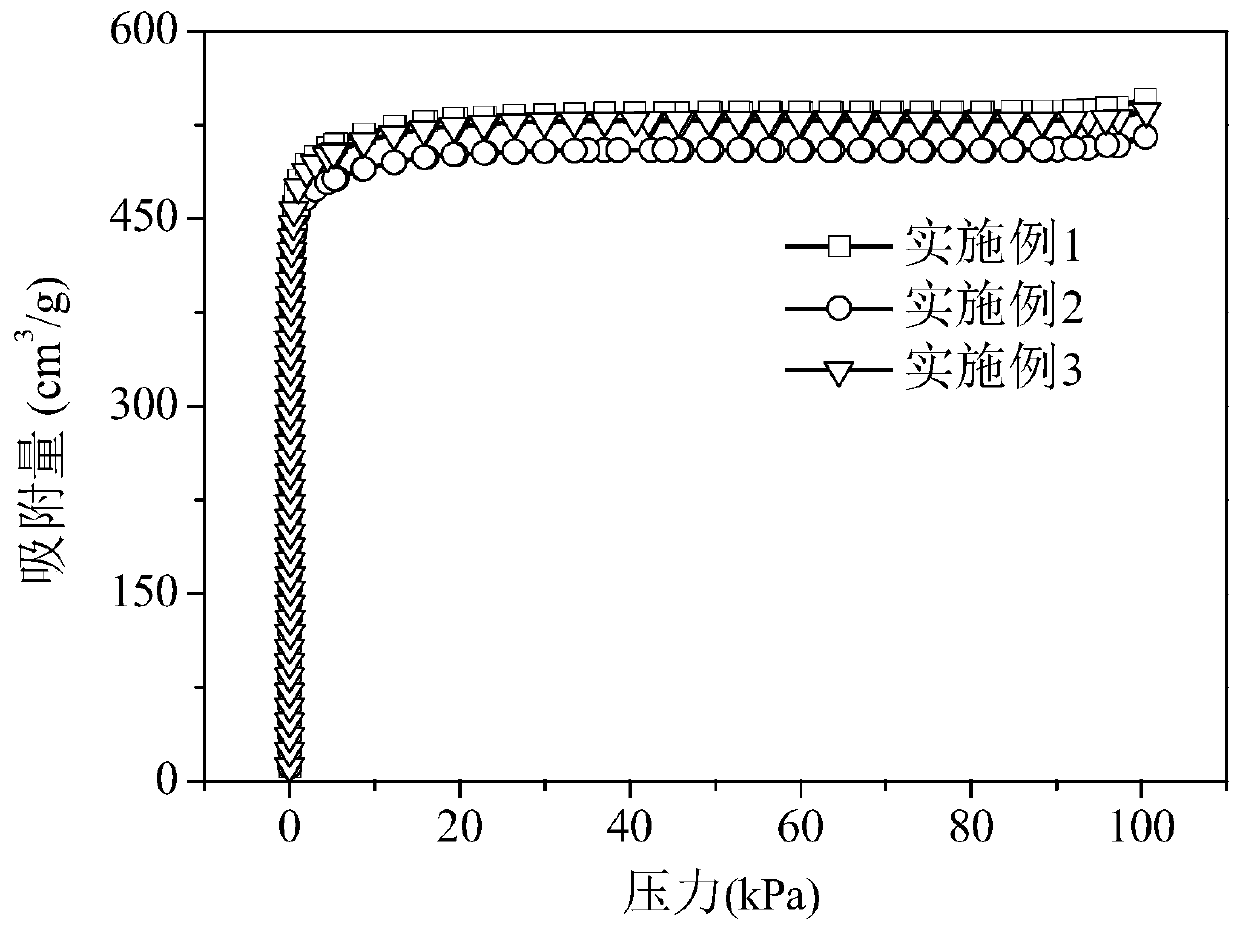
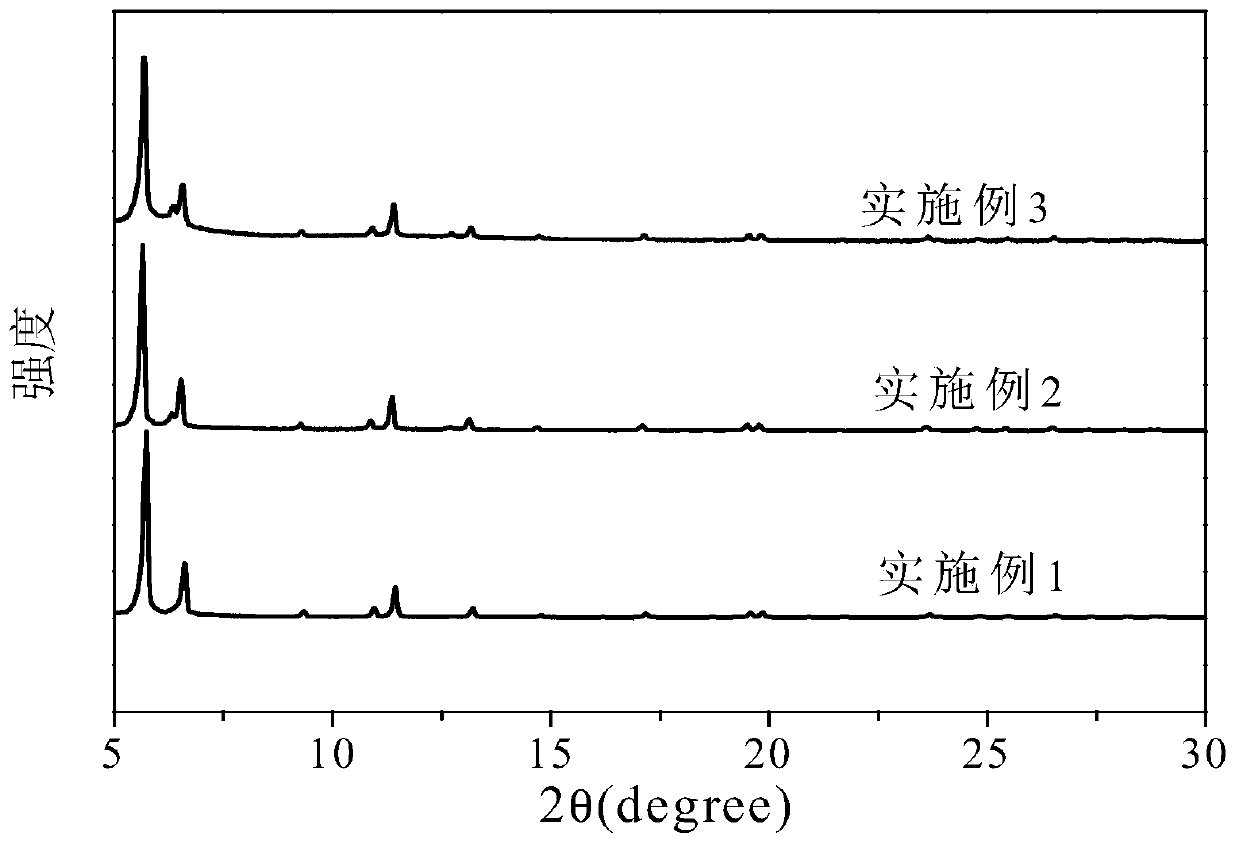
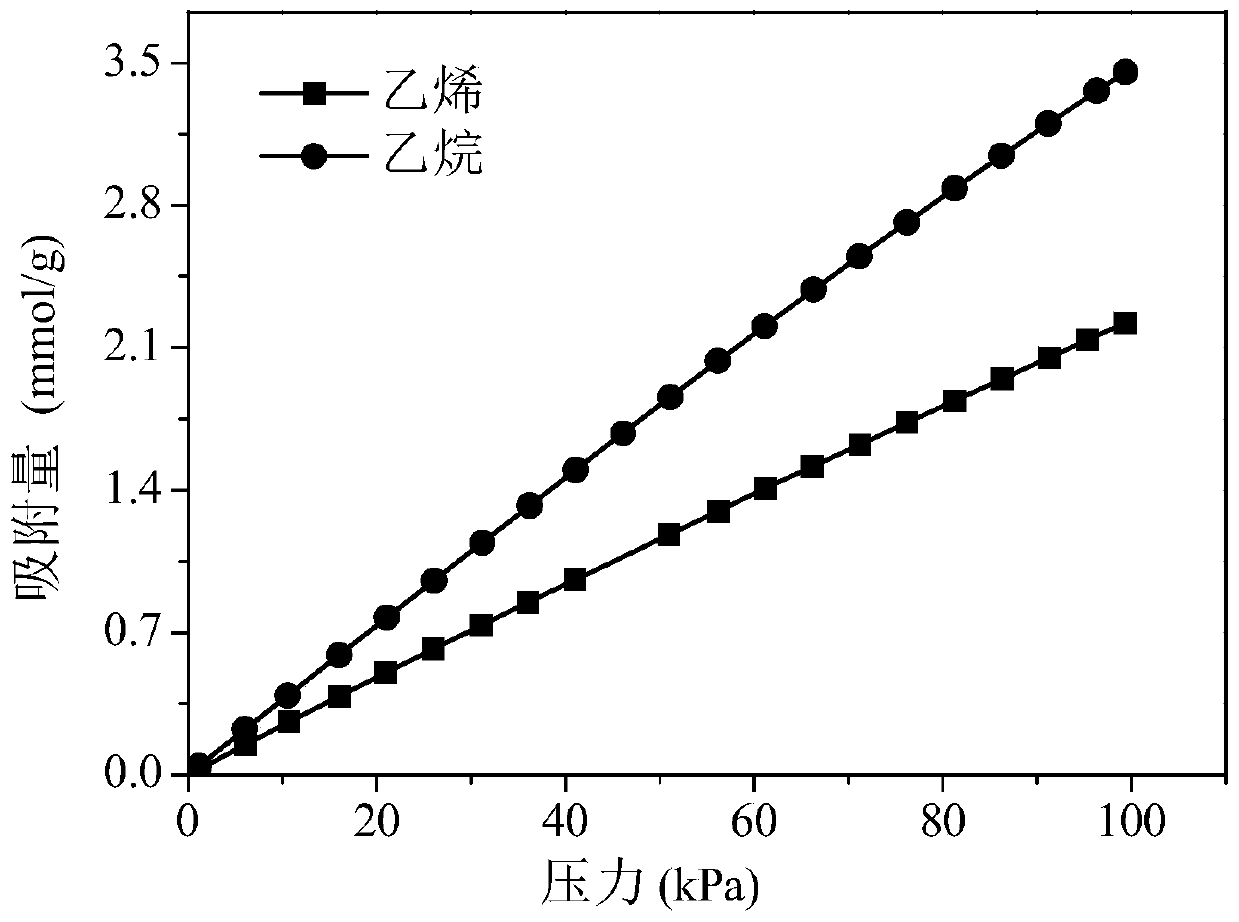
Preparation method for metal organic framework and carbon nitride composite material capable of preferentially adsorbing alkane
A metal-organic framework, preferential adsorption technology, applied in the direction of alkali metal compounds, organic chemistry, adsorption purification/separation, etc., to achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The ground graphite-like phase g-C 3 N 4 (2.54mg), ZrCl 4 (245mg1.05mmol), biphenyldicarboxylic acid (260mg1.05mmol), glacial acetic acid 2ml, N,N-dimethylformamide (DMF) 40ml, were added to the reaction kettle, stirred and sonicated for 20min. Then the reactor was heated to 100°C for 48h, then cooled to room temperature, and centrifuged. The product obtained by the reaction uses N,N-dimethylformyl
[0035] Rinse the material 3 times with amine; then soak in methanol for 48 hours, replace the methanol every 12 hours, and then keep it at 80°C for 6 hours. Finally, it was activated under vacuum condition at 100° C. for 12 hours to obtain the product, which was labeled as Example 1.
Embodiment 2
[0037] The ground graphite-like phase g-C 3 N 4 (5.08mg), ZrCl 4 (245mg1.05mmol), biphenyldicarboxylic acid (260mg1.05mmol), glacial acetic acid 2ml, N,N-dimethylformamide (DMF) 40ml were added to the reaction kettle, stirred and sonicated for 30min. Afterwards, the reactor was heated to 120° C. for 36 h, then cooled to room temperature, and centrifuged. The product obtained by the reaction was rinsed with N,N-dimethylformamide for 4 times; then soaked in methanol for 48 hours, the methanol was replaced every 12 hours, and then kept at 80°C for 6 hours. Finally, it was activated under vacuum at 120° C. for 8 hours to obtain the product, which was labeled as Example 2.
Embodiment 3
[0039] The ground graphite-like phase g-C 3 N 4 (15.23mg), ZrCl 4 (245mg1.05mmol), biphenyl dicarboxylic acid (260mg1.05mmol), 2ml of glacial acetic acid, 40ml of N,N-dimethylformamide (DMF) were added to the reaction kettle, stirred and ultrasonicated for 60min. Afterwards, the reactor was heated to 150° C. for 24 hours, then cooled to room temperature, and centrifuged. The product obtained by the reaction was rinsed with N,N-dimethylformamide for 5 times; then soaked in methanol for 48 hours, the methanol was replaced every 12 hours, and then kept at 80°C for 6 hours. Finally, the product was activated under vacuum condition at 160° C. for 4 hours to obtain the product, which was labeled as Example 3.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap