Histamine dihydrochloride injection and preparation method thereof
A technology of histamine dihydrochloride and injection, which is applied in the field of orthopedics, can solve the problems of affecting drug efficacy, safety issues, and easy shape changes, and achieves the effects of stable and feasible process, reduced safety concerns, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
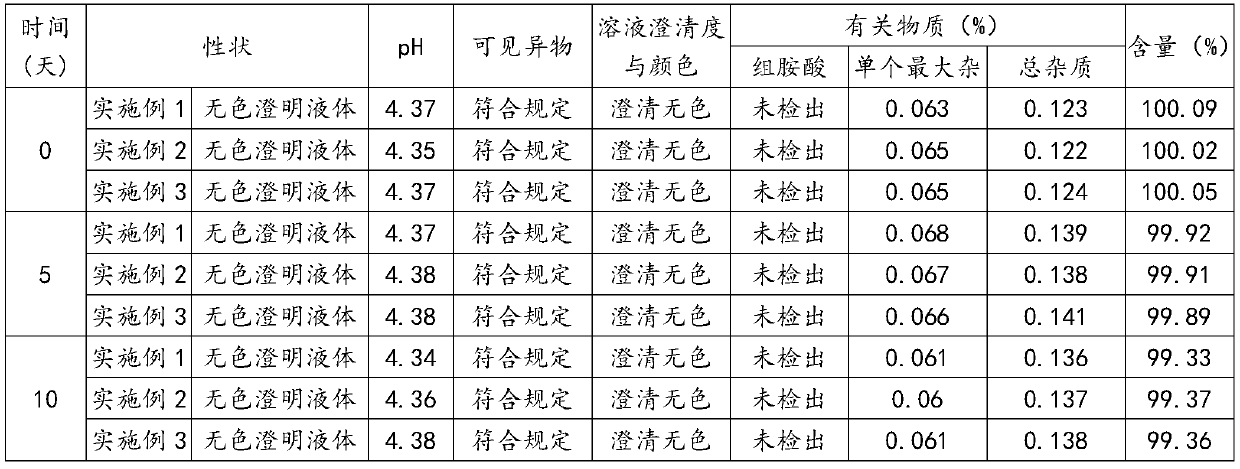
Examples
Embodiment 1
[0025] A histamine dihydrochloride injection is composed of histamine dihydrochloride, a pH regulator and water for injection.
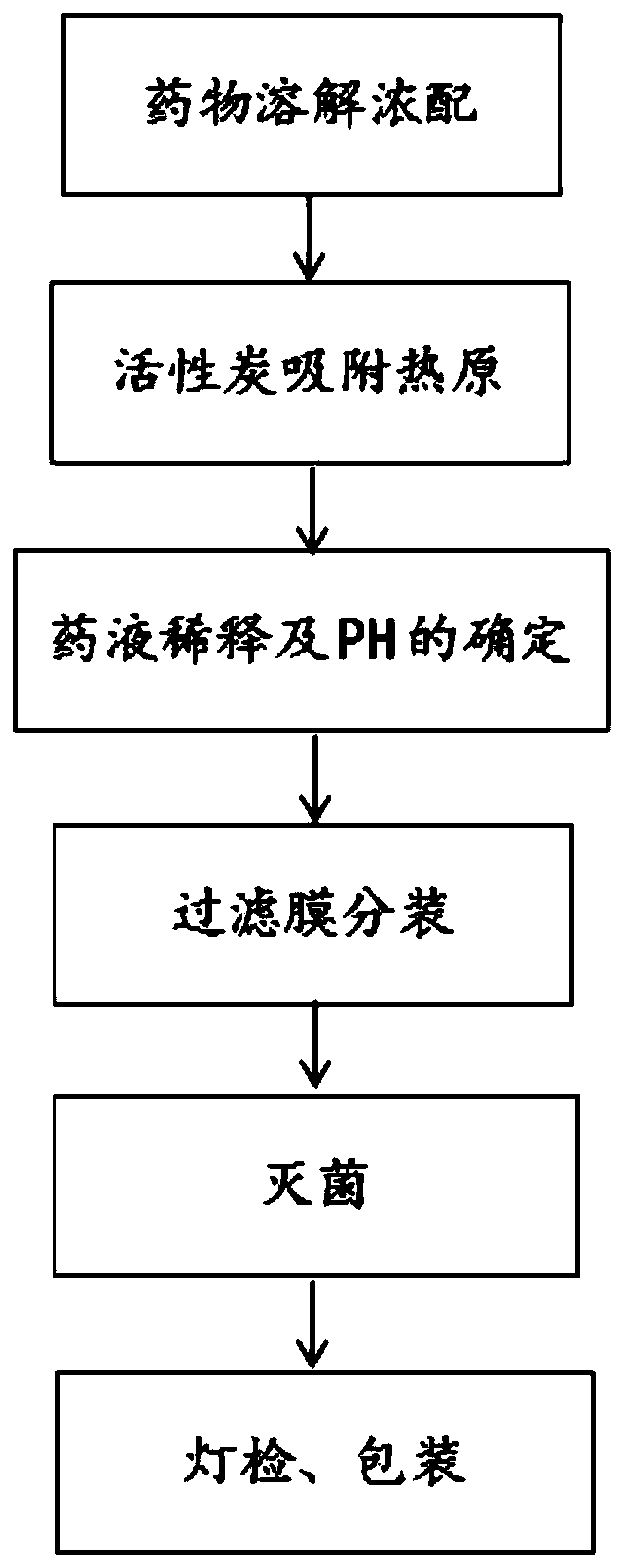
[0026] like figure 1 Shown a kind of preparation method of histamine dihydrochloride injection, comprises the steps:
[0027] S1, drug dissolution concentrated preparation: accurately weigh 0.505g of histamine dihydrochloride, add 50ml of water for injection, fully stir to dissolve it, and obtain a concentrated solution of histamine dihydrochloride with a concentration of about 10mg / ml;
[0028] S2, activated carbon adsorbs pyrogen: heat the concentrated solution of histamine dihydrochloride prepared in step S1 to 60°C in a water bath, then add 0.1% (W / V) activated carbon for needles, adsorb at a constant temperature of 60°C for 25 minutes, filter through a 0.22 μm filter Membrane decarburization;
[0029] S3, dilution of medicinal solution and determination of pH: adding water for injection to the solution after decarburization in step S2 and dilu...
Embodiment 2
[0034] A histamine dihydrochloride injection is composed of histamine dihydrochloride, a pH regulator and water for injection.
[0035] like figure 1 Shown a kind of preparation method of histamine dihydrochloride injection, comprises the steps:
[0036] S1, drug dissolution and concentrated preparation: accurately weigh 0.501g of histamine dihydrochloride, add 50ml of water for injection, fully stir to dissolve it, and obtain a concentrated solution of histamine dihydrochloride with a concentration of about 10mg / ml;
[0037] S2, activated carbon adsorbs pyrogen: heat the concentrated solution of histamine dihydrochloride prepared in step S1 to 60°C in a water bath, then add 0.1% (W / V) activated carbon for needles, adsorb at a constant temperature of 60°C for 28 minutes, filter through a 0.22 μm filter Membrane decarburization;
[0038] S3, dilution of medicinal solution and determination of pH: adding water for injection to the solution after decarburization in step S2 and ...
Embodiment 3
[0043] A histamine dihydrochloride injection is composed of histamine dihydrochloride, a pH regulator and water for injection.
[0044] like figure 1 Shown a kind of preparation method of histamine dihydrochloride injection, comprises the steps:
[0045] S1, drug dissolving concentrated preparation: accurately weigh 0.504g of histamine dihydrochloride, add 50ml of water for injection, fully stir to dissolve it, and obtain a concentrated solution of histamine dihydrochloride with a concentration of about 10mg / ml;
[0046] S2, activated carbon adsorbs pyrogen: heat the concentrated solution of histamine dihydrochloride prepared in step S1 to 60°C in a water bath, then add 0.1% (W / V) activated carbon for needles, adsorb at a constant temperature of 60°C for 30min, filter through a 0.22 μm filter Membrane decarburization;
[0047] S3, dilution of medicinal solution and determination of pH: adding water for injection to the solution after decarburization in step S2 and diluting t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com