1,4-diionic sulfur-containing ylide derivative based on asymmetric alkyne, and preparation method thereof
An ionic and asymmetric technology, applied in organic chemistry and other directions, can solve the problems of limiting the research value and application scope of ylide salts, and achieve the effects of good yield, easy amplification and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The reaction formula of Example 1, the specifically used compound IV-1 and compound V-1 and the structure of the product I-1 are as follows.
[0023]
[0024] The specific experimental steps are: 791 mg (10 mmol, 1.0 equivalent) of compound II-1 and 320 mg (10 mmol, 1.0 equivalent) of compound III are dissolved in 50 mL of dichloromethane solvent, and 166 mg (10 mmol, 1.0 equivalent) of compound IV-1, naturally rose to room temperature, and reacted for 24 hours. After the reaction, the target compound I-1 can be obtained by suction filtration.
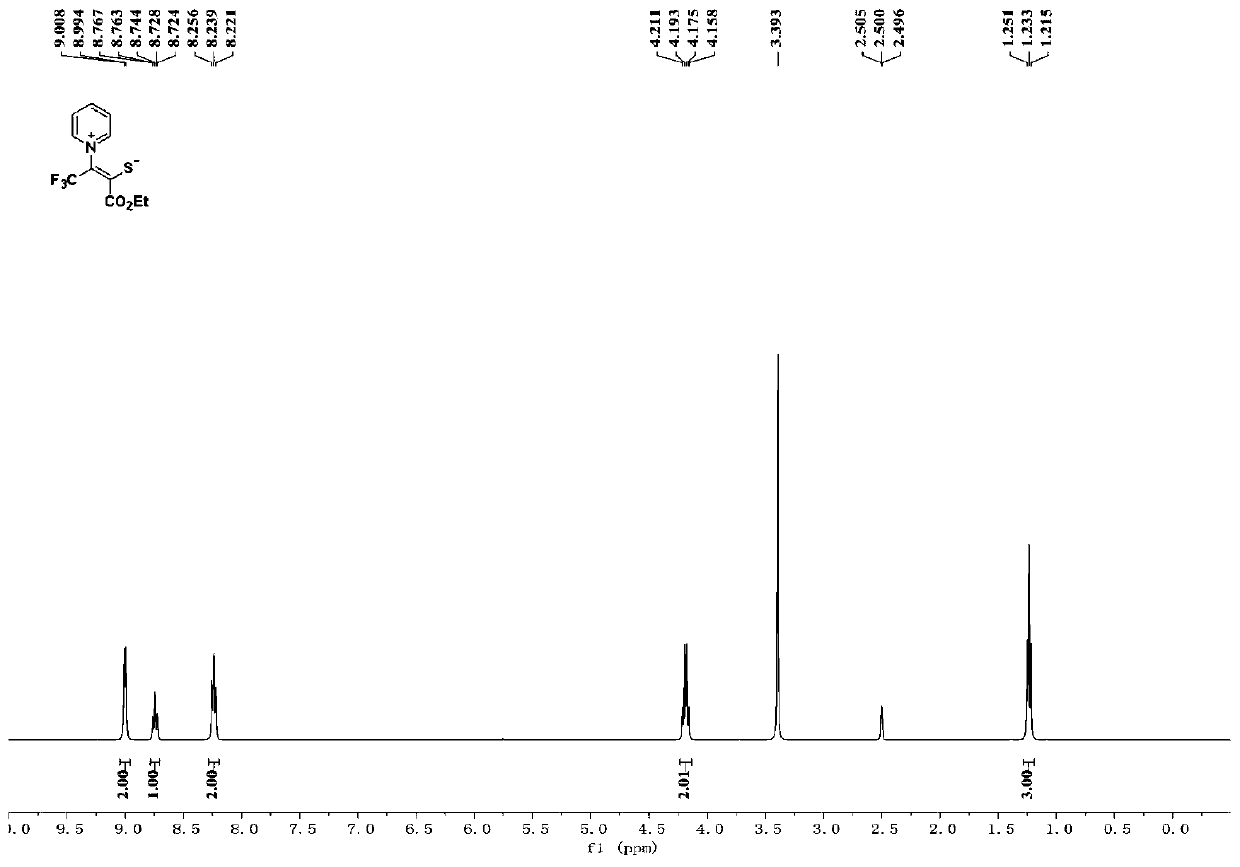
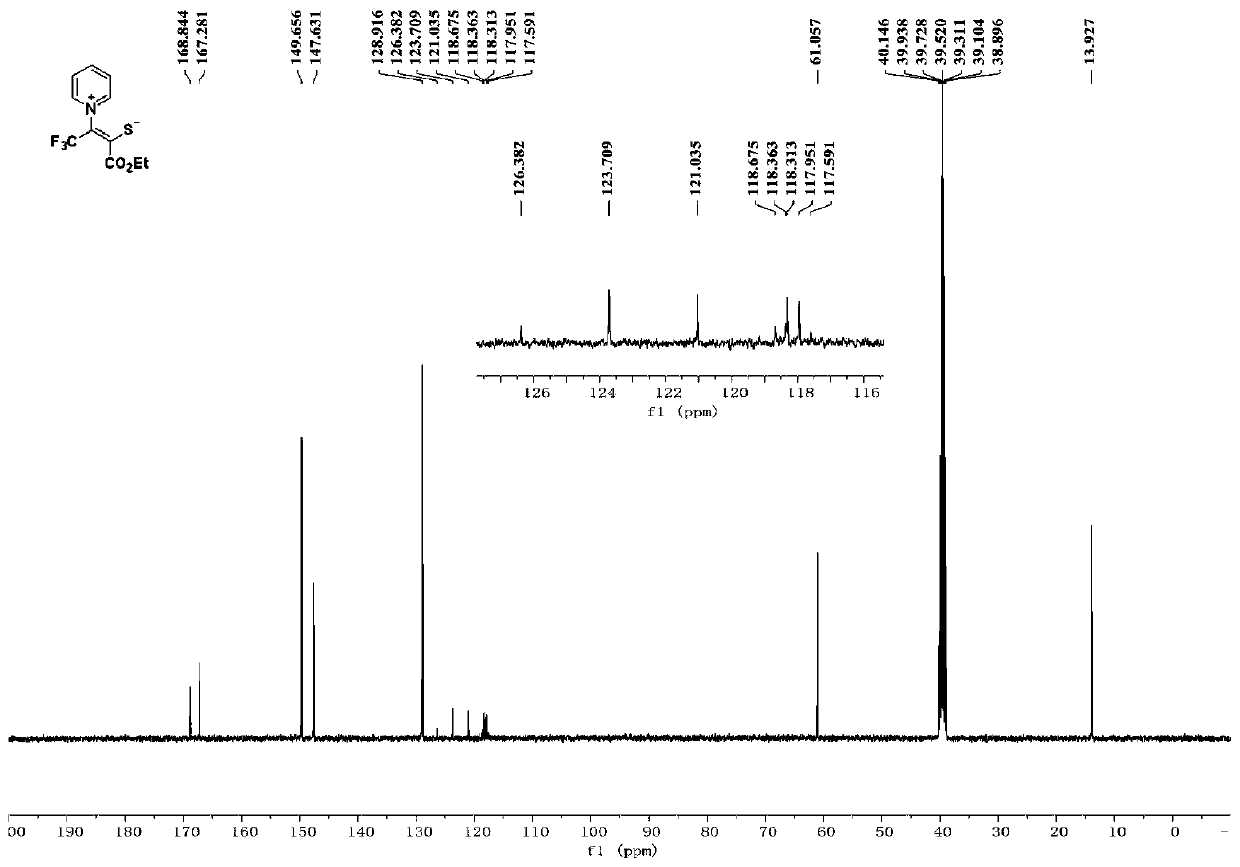
[0025] Product Ⅰ-1 is a yellow solid with a yield of 58%; melting point: 118-119°C. 1 H NMR (400MHz, d 6 -DMSO) δ9.00(d, J=5.6Hz, 2H), 8.74(t, J=7.6Hz, 1H), 8.24(t, J=7.2Hz, 2H), 4.18(q, J=7.2Hz, 2H), 1.23(t, J=7.1Hz, 3H); 13 C NMR (100MHz, d 6 -DMSO) δ168.84, 167.28, 149.66, 147.63, 128.92, 122.4(q, J=267.3Hz), 118.1(q, J=36.2Hz), 61.06, 13.93; ESI-HRMS m / z calcdfor C 11 h 10 f 3 NO 2 S+H + 278.0457,found 278.0453....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com