Device for high-temperature chlorination dehydrogenating of alkane gases and use method
A technology for high-temperature chlorination and alkanes, which is applied in the field of high-temperature chlorination and dehydrogenation of alkane gases. It can solve the problems of poor mass and heat transfer effects and unstable yields, and achieve improved mass transfer performance and improved heat transfer. performance, effect of increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
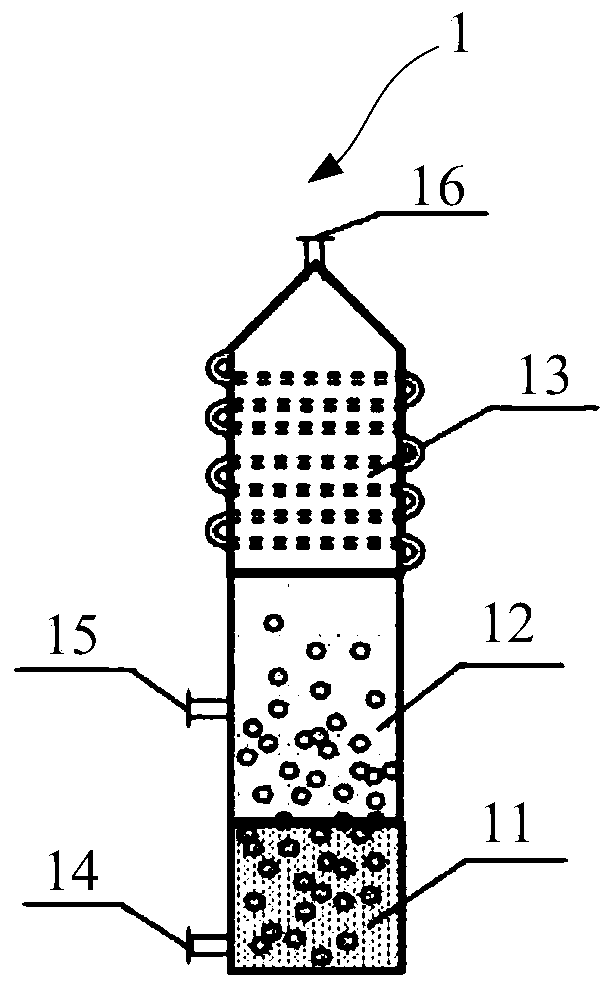
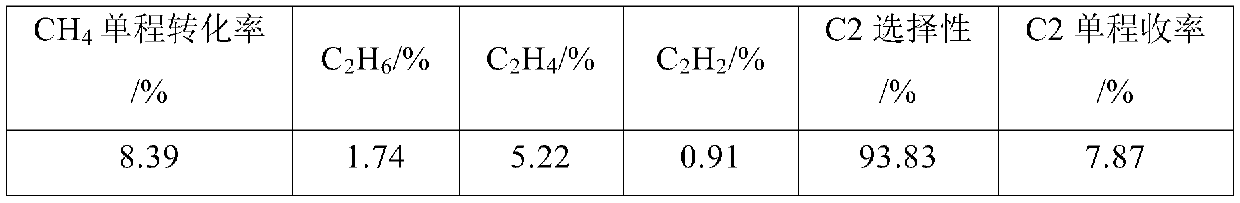
[0074] (1) Cl 2 The chlorine gas inlet of the low-melting-point molten metal layer at the bottom of the tower is passed into the metal Pb molten layer, and the temperature of the low-melting-point molten metal layer is controlled to be 950°C, so that Cl 2 React with metal Pb to get PbCl 2 steam, PbCl 2 The steam enters the chloride molten salt layer composed of CuCl-KCl (the molar ratio of CuCl to KCl is 1:1);
[0075] (2) CH 4 The gas is passed into the dissolved PbCl from the alkane gas inlet of the molten salt layer 2 Molten salt layer composed of steam CuCl-KCl, controlling CH 4 With step 1) the Cl that passes into 2 The molar ratio is 5:1, the temperature of the molten salt layer is controlled at 950°C, so that CH 4 with PbCl 2 Chloride dehydrogenation coupling to get the crude product gas, PbCl 2 Reduced to liquid Pb back to the metal melt layer, and continue with Cl 2reaction.
[0076] (3) control the temperature of the top gas-liquid separation layer cooling ...
Embodiment 2
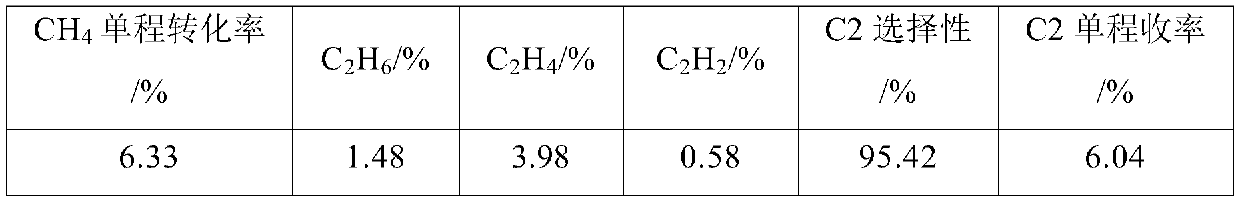
[0080] (1) Cl 2 The chlorine gas inlet of the low-melting-point metal melt layer at the bottom of the tower is passed into the metal Sn melt layer, and the temperature of the low-melting-point metal melt layer is controlled to be 870°C, so that Cl 2 React with metal Sn to get SnCl 2 Steam, SnCl 2 Steam enters with CuCl-CsCl-CaCl 2 (CuCl, CsCl and CaCl 2 A chloride molten salt layer composed of a molar ratio of 1:0.1:0.1);
[0081] (2) CH 4 The gas is passed into the dissolved SnCl from the alkane gas inlet of the molten salt layer 2 Vapor CuCl-CsCl-CaCl 2 Composition of the molten salt layer that controls the CH 4 With step 1) the Cl that passes into 2 The molar ratio is 10:1, the temperature of the molten salt layer is controlled at 850°C, so that CH 4 with SnCl 2 Chlorination dehydrogenation coupling to get the crude product gas, SnCl 2 Reducted into liquid Sn and returned to the molten metal layer, and continued with Cl 2 reaction.
[0082] (3) Control the temp...
Embodiment 3
[0086] (1) Cl 2 Lead into the metal Bi melt layer from the chlorine gas inlet of the low melting point metal melt layer at the bottom of the tower, and control the temperature of the low melting point metal melt layer to be 700 ° C, so that Cl 2 React with metal Bi to get BiCl 3 steam, BiCl 3 Steam enters with KCl-ZnCl 2 (KCl and ZnCl 2 A chloride molten salt layer composed of a molar ratio of 1:0.5);
[0087] (2) CH 4 The gas is passed into the dissolved BiCl from the alkane gas inlet of the molten salt layer 3 Vapor KCl-ZnCl 2 Composition of the molten salt layer that controls the CH 4 With step 1) the Cl that passes into 2 The molar ratio is 1:1, and the temperature of the molten salt layer is controlled at 700°C so that CH 4 with BiCl 3 Chlorination dehydrogenation coupling to get the crude product gas, BiCl 3 Restore to liquid Bi and return to the metal melt layer, and continue to work with Cl 2 reaction.
[0088] (3) Control the temperature of the gas-liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com