Oxygen-substituted phenylimidazole XOR/URAT1 dual inhibitor, preparation and applications thereof
A dual inhibitor, phenylimidazole technology, applied in the field of oxygen-substituted phenylimidazole XOR/URAT1 dual inhibitors and its preparation and application, to achieve excellent inhibitory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
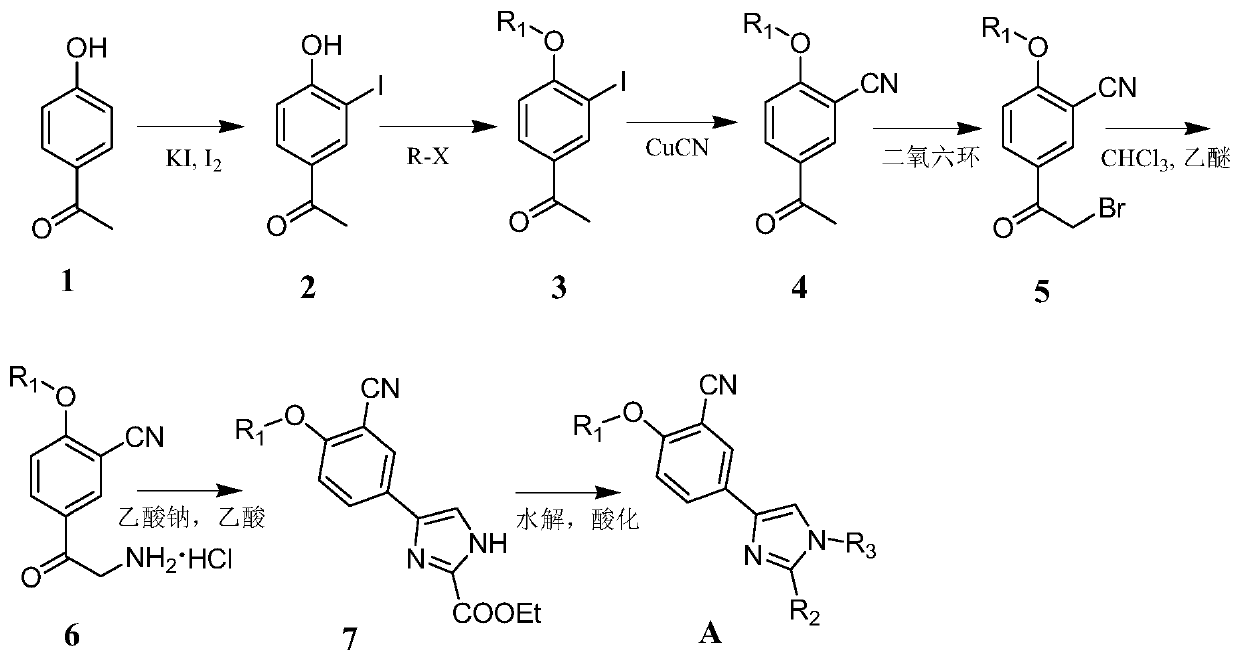
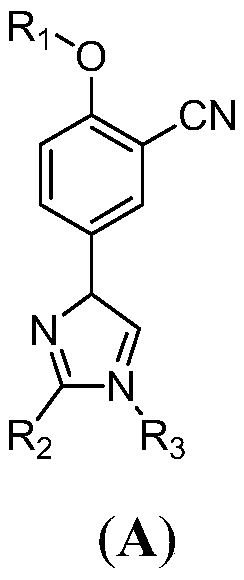
[0045] 4-(3-cyano-4-ethoxy-phenyl)-1H-imidazole-2-carboxylic acid (A 1 )Synthesis:
[0046] (1) After dissolving p-hydroxyacetophenone (1, 2.0g, 14.7mmol) in aqueous ammonia, add KI (12g, 73.5mmol) and I 2 (3.7g, 14.7mmol) into an aqueous solution. Stir the reaction at room temperature overnight, after TLC traced the complete reaction, filter, and the filtrate was acidified with concentrated hydrochloric acid, filtered to obtain a yellow precipitate, and the crude product was recrystallized with methanol-water=7:3 to obtain 3-iodo-4-hydroxy-acetophenone (2 )3.13g, yield 80.7%.
[0047] (2) 3-iodo-4 hydroxy-acetophenone (2, 4.0g, 15.3mmol), K 2 CO 3 (6.3g, 45.8mmol) was added to the flask, then DMF was added, stirred at room temperature for 0.5h, then ethyl iodide (7.1g, 45.8mmol) was added, reacted at 80°C, and the reaction was complete after 3h. After adding water to dilute, add ethyl acetate to extract 3 times, wash 2 times with water, then wash 2 times with saturated b...
Embodiment 2
[0057] 4-(3-cyano-4-isopropoxy-phenyl)-1H-imidazole-2-carboxylic acid (A 2 )Synthesis:
[0058] (1) After dissolving p-hydroxyacetophenone (1, 2.0g, 14.7mmol) in ammonia water, add the configured KI (12g, 73.5mmol) and I 2 (3.7g, 14.7mmol) in aqueous solution. Stir the reaction at room temperature overnight, after TLC traced the complete reaction, filter, and the filtrate was acidified with concentrated hydrochloric acid, filtered to obtain a yellow precipitate, and the crude product was recrystallized with methanol-water=7:3 to obtain 3-iodo-4-hydroxy-acetophenone (2 )3.13g, yield 80.7%.
[0059] (2) 3-iodo-4 hydroxy-acetophenone (2, 4.0 g, 15.3 mmol), K 2 CO 3 (6.3g, 45.8mmol) and 20mL of DMF were added to the flask, stirred at room temperature for 0.5h, added iodoisopropane (7.8g, 45.8mmol), reacted at 80°C, and the reaction was complete after 3h. Add 150 mL of water to dilute, extract with ethyl acetate (100 mL×3), wash twice with water, then wash twice with saturated...
Embodiment 3
[0069] 4-(3-cyano-4-isobutoxy-phenyl)-1H-imidazole-2-carboxylic acid ethyl ester (A 3 )Synthesis:
[0070] (1) Dissolve p-hydroxyacetophenone (1, 2.0g, 14.7mmol) in aqueous ammonia, add to the mixture containing KI (12g, 73.5mmol) and I 2 (3.7g, 14.7mmol) into an aqueous solution. Stir the reaction at room temperature overnight, after TLC traced the complete reaction, filter, and the filtrate was acidified with concentrated hydrochloric acid, filtered to obtain a yellow precipitate, and the crude product was recrystallized with methanol-water=7:3 to obtain 3-iodo-4-hydroxy-acetophenone (2 )3.13g, yield 80.7%.
[0071] (2) 3-iodo-4 hydroxy-acetophenone (2, 4.0g, 15.3mmol), K 2 CO 3 (6.3g, 45.8mmol) and 20mL DMF were added to the flask, stirred at room temperature for 0.5h, and bromoisobutane (6.3g, 45.8mmol) was added, and reacted at 85°C, and the reaction was complete after 3h. Add 150 mL of water to dilute, extract with ethyl acetate (100 mL×3), wash twice with water, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com