Preparation method of bimetallic MOF derived oxygen evolution electrocatalyst
An electrocatalyst, bimetallic technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as inability to meet industrial applications, poor conductivity, etc., and achieve good application prospects, excellent performance, Prepare simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
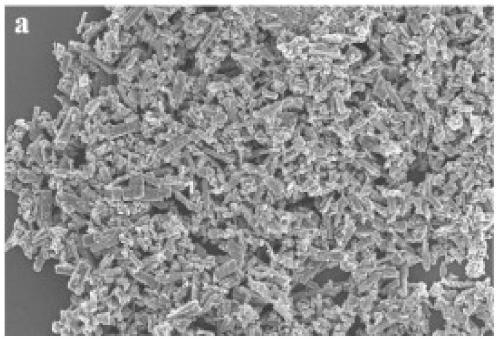
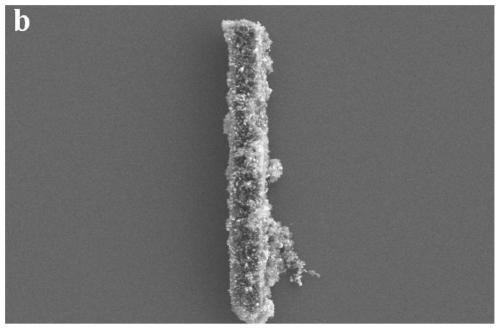
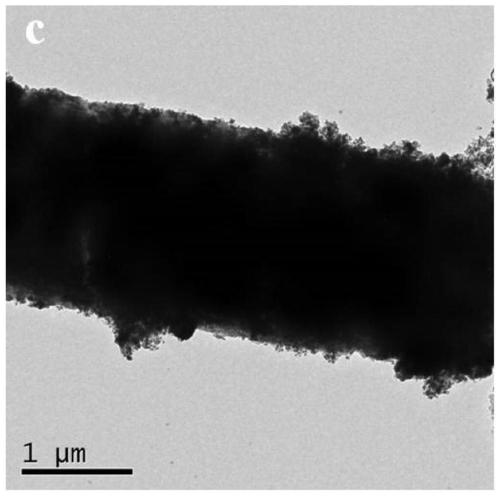
Image
Examples
Embodiment 1
[0044] Table 1 Fe prepared with different molar ratios of Fe and Ni y Ni 4-y C x catalyst
[0045] Catalyst name Molar ratio of Fe to Ni Fe 4 Ni 0 C x
4:0.01 Fe 3 Ni 1 C x
3:1 Fe 2 Ni 2 C x
2:2 Fe 1 Ni 3 C x
1:3 Fe 0 Ni 4 C x
0.01:4
[0046] Fe and Ni molar ratios of different Fe and Ni molar ratios as shown in Table 1 were prepared respectively. y Ni 4-y C x Catalyst, Fe when L-glutamic acid is 1mmol and trimesic acid is 1.34mmol 1 Ni 3 C x For example, the specific preparation process is as follows:
[0047] (1) 1mmol Fe(CH 3 COO) 2 , 3mmol Ni(CH 3 COO) 2 and 1mmol L-glutamic acid were added to 80mL deionized water and stirred rapidly to dissolve completely, marked as A 1 solution; at the same time, add 1.34mmol trimesic acid into 80mL absolute ethanol and stir rapidly to dissolve it completely, marked as B 1 solution. Subsequently, the B 1 The solution is quickly poured int...
Embodiment 2
[0054] Table 2 Fe using different molar amounts of L-glutamic acid y Ni 4-y C x catalyst
[0055] Catalyst name Molar amount of L-glutamic acid (mmol) Fe 1 Ni 3 C x -0(0.01mmol)
0.01 Fe 1 Ni 3 C x -1(1mmol)
1 Fe 1 Ni 3 C x -2(2mmol)
2
[0056] Prepare the Fe of L-glutamic acid of different consumptions as shown in table 2 respectively y Ni 4-y C x Catalyst, with Fe(CH 3 COO) 2 1mmol, Ni(CH 3 COO) 2 3mmol, Fe when trimesic acid is 1.34mmol 1 Ni 3 C x -1 (1mmol) as an example, the specific preparation process is as follows:
[0057] (1) 1mmol Fe(CH 3 COO) 2 , 3mmol Ni(CH 3 COO) 2 and 1mmol L-glutamic acid were added to 80mL deionized water and stirred rapidly to dissolve completely, marked as A 2 solution; at the same time, add 1.34mmol trimesic acid into 80mL absolute ethanol and stir rapidly to dissolve it completely, marked as B 2 solution. Subsequently, the B 2 The solution is quickly poured into A...
Embodiment 3
[0063] Table 3 Fe prepared by using different molar amounts of trimesic acid y Ni 4-y C x catalyst
[0064] Catalyst name Molar amount of trimesic acid (mmol) Fe 1 Ni 3 C x -0.67 (0.67mmol)
0.67 Fe 1 Ni 3 C x -1.34 (1.34mmol)
1.34 Fe 1 Ni 3 C x -2.01 (2.01mmol)
2.01
[0065] Prepare the Fe of trimesic acid in different amounts as shown in table 3 respectively y Ni 4-y C x Catalyst, with Fe(CH 3 COO) 2 1mmol, Ni(CH 3 COO) 2 3mmol, Fe when L-glutamic acid is 1mmol 1 Ni 3 C x -1.34 (1.34mmol) as an example, the specific preparation process is as follows:
[0066] (1) 1mmol Fe(CH 3 COO) 2 , 3mmol Ni(CH 3 COO) 2 and 1mmol L-glutamic acid were added to 80mL deionized water and stirred rapidly to dissolve completely, marked as A 3 solution; at the same time, add 1.34mmol trimesic acid into 80mL absolute ethanol and stir rapidly to dissolve it completely, marked as B 3 solution. Subsequently, the B 3 The sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com