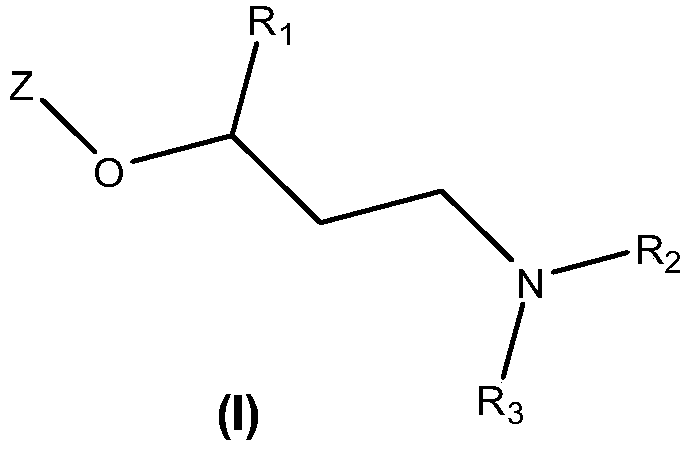
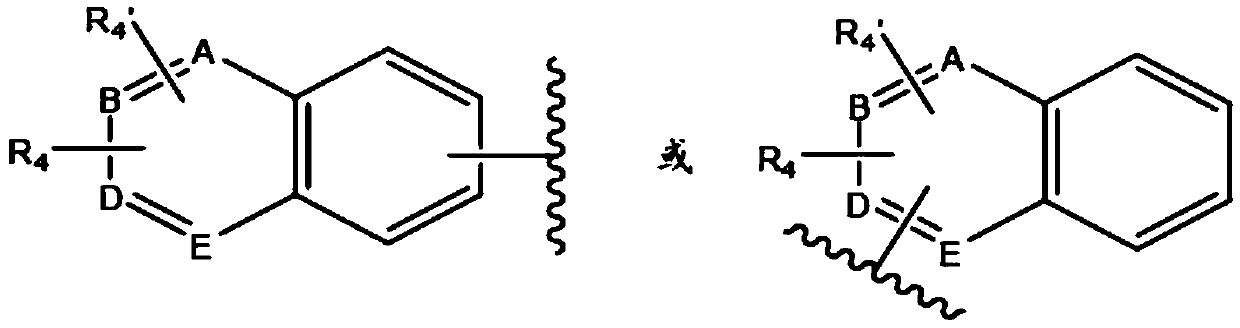
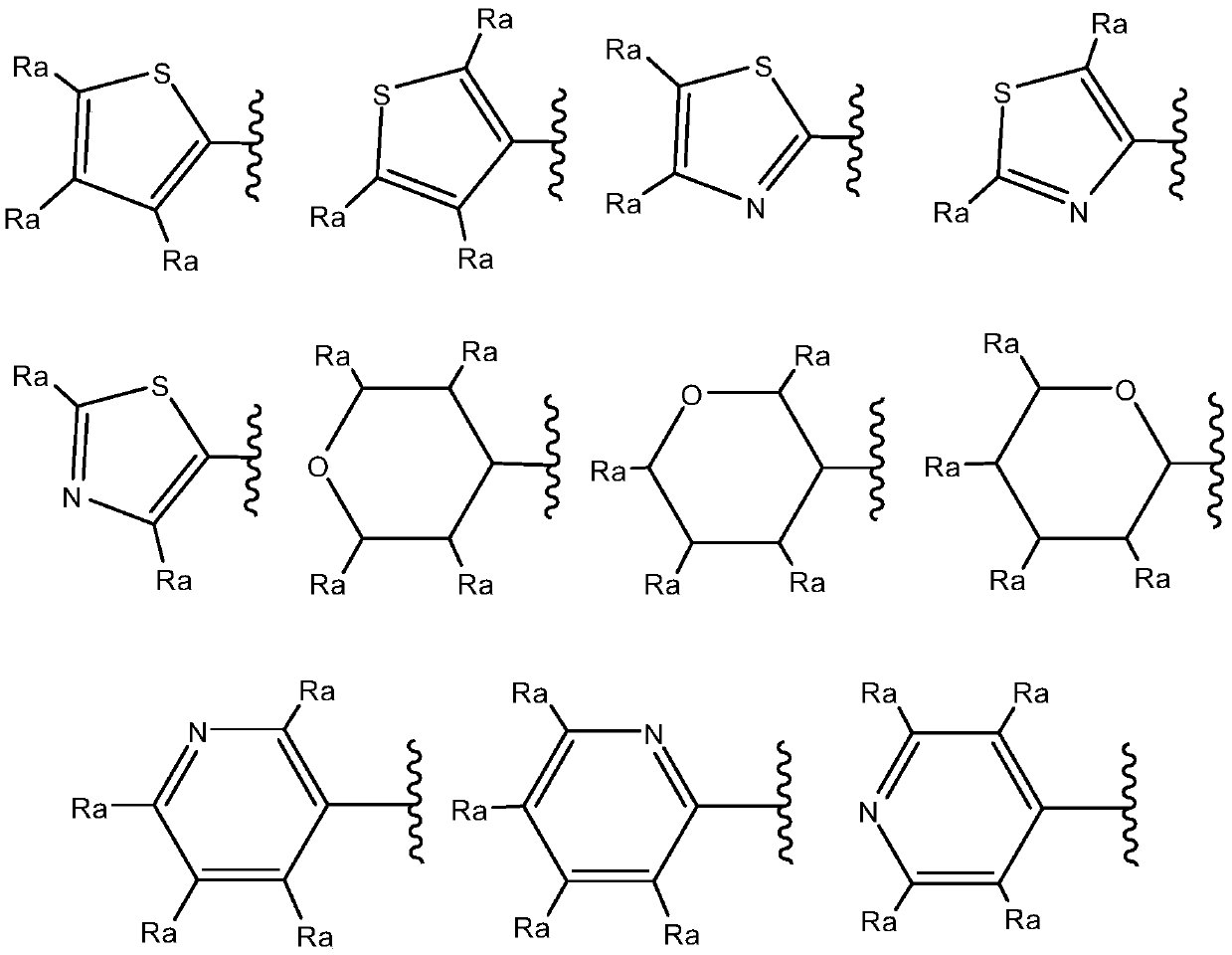
New quinoline and isoquinoline derivatives for treating pain and pain related conditions
A technology for isoquinoline and compounds, applied in the field of preparing said compounds, capable of solving problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0287] The preparation of compounds of general formula IV can be carried out according to several methods described in the literature. As an example, two synthetic routes are described in Scheme 2:
[0288] Scenario 2
[0289]
[0290] where R 1 , R 2 and R 3 has the meaning defined above for the compound of formula (I).
[0291] Following Route A, a compound of formula (VIII) is treated with a strong base such as butyllithium to generate the corresponding organometallic reagent, followed by condensation with the Weinreb amide of formula (VII) in a suitable solvent such as tetrahydrofuran, Compounds of formula (IV) are obtained.
[0292] Alternatively, compounds of formula (IV) can be prepared by Mannich reaction of acetyl compounds of formula (IX) with amines of formula (VI) and a source of formaldehyde such as paraformaldehyde, preferably in The condensation is carried out in the presence of an acid such as hydrochloric acid in a suitable solvent such as ethanol o...
example
[0347] In the following preparation examples, the synthesis of two intermediate derivatives and compounds according to the invention is disclosed.
[0348] The following abbreviations are used in the examples:
[0349] ACN: Acetonitrile
[0350] ADDP: 1,1′-(Azodicarbonyl)dipiperidine
[0351] Boc: tert-butoxycarbonyl
[0352] BuLi: Butyl Lithium
[0353] Conc: concentrated
[0354] DCM: dichloromethane
[0355] DEA: Diethylamine
[0356] DIAD: Diisopropyl azodicarboxylate
[0357] DMA: N,N-Dimethylacetamide
[0358] Eq: Equivalent
[0359] Et 2 O: Ether
[0360] EtOAc: ethyl acetate
[0361] EtOH: ethanol
[0362] EX: Example
[0363] h: hours
[0364] HPLC: High Performance Liquid Chromatography
[0365] 2-Me-CBS-oxazoborin: 5,5-biphenyl-2-methyl-3,4-propanol-1,3,2-oxazoborin (Corey-Bakshi-Shibata oxazaborin catalyst)
[0366] MeOH: Methanol
[0367] MS: mass spectrometry
[0368] min minutes
[0369] PPh 3 :Triphenylphosphine
[0370] Quant: quantitativ...
example 1
[0446] Example 1: N-methyl-3-(quinolin-8-yloxy)-3-(thiophen-2-yl)propan-1-amine
[0447]
[0448] Step 1.8-(3-Chloro-1-(thiophen-2-yl)propoxy)quinoline: To 3-chloro-1-(thiophen-2-yl)propan-1-ol (0.2 g, 1.13 mmol) , tributylphosphine (0.34mL, 1.36mmol) and quinolin-8-ol (0.164g, 1.13mmol) in toluene (6mL) was added ADDP (0.343g, 1.36mmol), and the reaction mixture was heated at 100 °C overnight. Then it was allowed to cool, the suspension was filtered and the collected solid was washed with toluene. The filtrate containing the desired product was concentrated in vacuo. The crude product was purified by flash silica gel chromatography (gradient: DCM to MeOH:DCM (1:4)) to afford the title compound (74 mg, 21% yield).
[0449] Step 2. The title compound: In a sealed tube, the product obtained in Step 1 (74mg, 0.244mmol), NaI (7.3mg, 0.049mmol) and methylamine (33wt% in EtOH, 0.152mL, 1.22mmol) were mixed in The mixture in EtOH (28 mL) was heated at 100 °C overnight. Then, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com