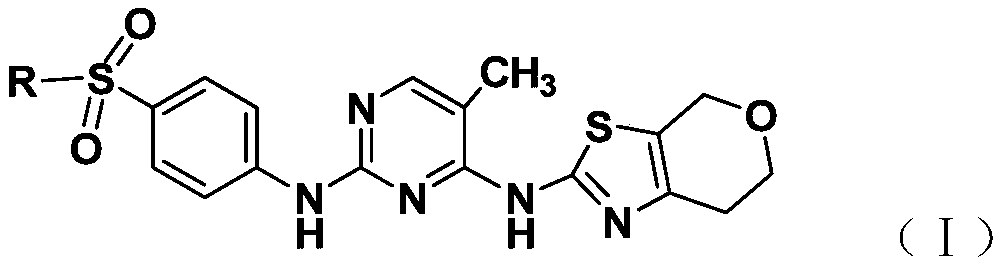
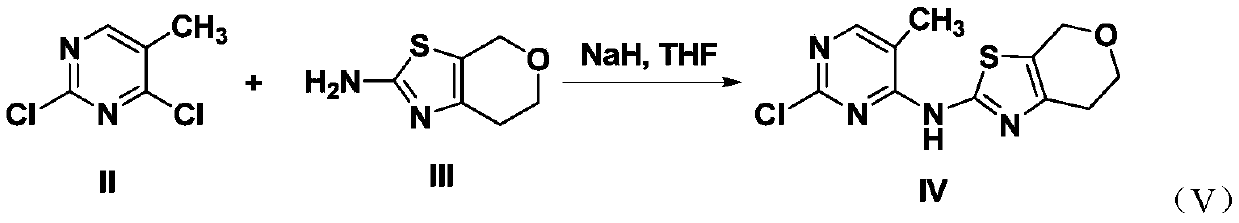
2,4-diaminopyrimidine containing dihydropyran thiazole and application of 2,4-diaminopyrimidine
A technology of dihydropyranothiazole and diaminopyrimidine, which is applied in the field of 2,4-diaminopyrimidine and can solve the problems of unsatisfactory effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 [preparation compound N 2 -(4-sulfonamidophenyl)-N 4 -(6,7-Dihydro-4H-pyrano[4,3-d]thiazol-2-yl)-5-methyl-2,4-diaminopyrimidine (Ia)]
[0023] 2.2mmol N 4 -(6,7-dihydro-4H-pyrano[4,3-d]thiazol-2-yl)-2-chloro-5-methylpyrimidine, 2.0mmol 4-sulfonamidoaniline and 4.4mmol Add aluminum chloride hydrochloride to 15ml of anhydrous ethylene glycol methyl ether, stir in ice bath for 1 hour, add 2.2mmol DIPEA, continue stirring in ice bath for 30 minutes, then start to heat up to 120°C for 15-20 hours. TLC detection, after the reaction, the reaction solution was poured into 100mL water, extracted with ethyl acetate (100mL × 3), left to separate the liquid, the organic phase was washed with saturated brine (80mL × 3), and then washed with anhydrous magnesium sulfate Dry, filter with suction, and remove ethyl acetate under reduced pressure to obtain a white solid, which is subjected to column chromatography with petroleum ether: ethyl acetate to obtain 0.135 g of wh...
Embodiment 2
[0025] Embodiment 2 [preparation N 2 -(4-Methanesulfonylphenyl)-N 4 -(6,7-dihydro-4H-pyrano[4,3-d]thiazol-2-yl)-5-methyl-2,4-diaminopyrimidine (Ib)]
[0026] 2.2mmol N 4 -(6,7-dihydro-4H-pyrano[4,3-d]thiazol-2-yl)-2-chloro-5-methylpyrimidine, 2.0mmol 4-methanesulfonylanilide and 4.4mmol Add aluminum chloride hydrochloride to 15ml of anhydrous ethylene glycol methyl ether, stir in ice bath for 1 hour, add 2.2mmol DIPEA, continue stirring in ice bath for 30 minutes, then start to heat up to 120°C for 15-20 hours. TLC detection, after the reaction, the reaction solution was poured into 100mL water, extracted with ethyl acetate (100mL × 3), left to separate the liquid, the organic phase was washed with saturated brine (80mL × 3), and then washed with anhydrous magnesium sulfate Dry, filter with suction, and remove ethyl acetate under reduced pressure to obtain a white solid, which is subjected to column chromatography with petroleum ether: ethyl acetate to obtain 0.129 g of whi...
Embodiment 3
[0028] Embodiment 3 (antitumor activity research)
[0029] The antitumor activity of the compounds of the present invention is demonstrated by the following method test. These effects indicate that the compounds of the present invention are useful in the treatment of cancer, especially solid tumors such as colon cancer, cervical cancer and liver cancer. The specific test method is as follows:
[0030] The in vitro antitumor activity of the compounds prepared in Examples 1-4 (numbered sequentially Ia-Id) was detected by MTT method. Collect logarithmic phase cells, adjust the concentration of cell suspension to 4×10 3 -5×10 3 cells / mL were inoculated in 96-well plates and incubated for 12-24 hours. After the cells adhered to the wall, drugs of different concentrations were added, and a total of 6 concentration gradients of 1.875, 3.75, 7.5, 15, 30, and 60 μmol / l were set, with 4 replicate wells for each concentration. Set at 37°C, 5% CO 2 Incubator, start timing cultivatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com