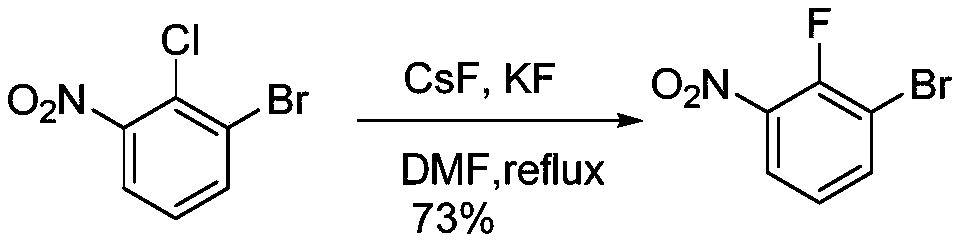
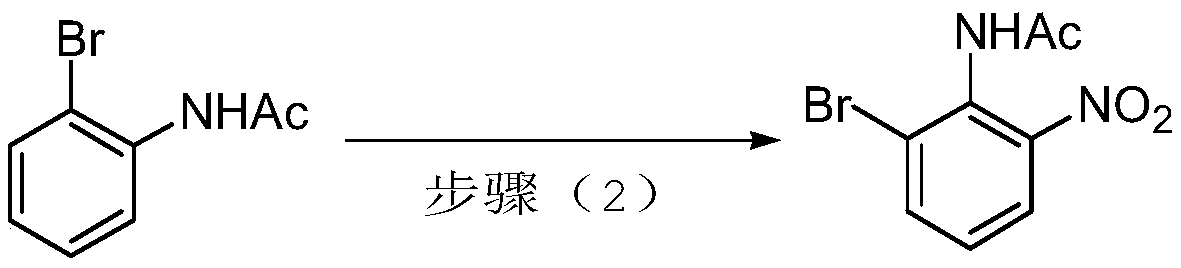
3-bromo-2-fluoronitrobenzene preparation method
A technology of fluoronitrobenzene and nitrophenyl, which is applied in the field of preparation of 3-bromo-2-fluoronitrobenzene, can solve the problems of low product purity, difficult to purify, unsuitable for industrial production and the like, and achieves high product purity , high conversion rate, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
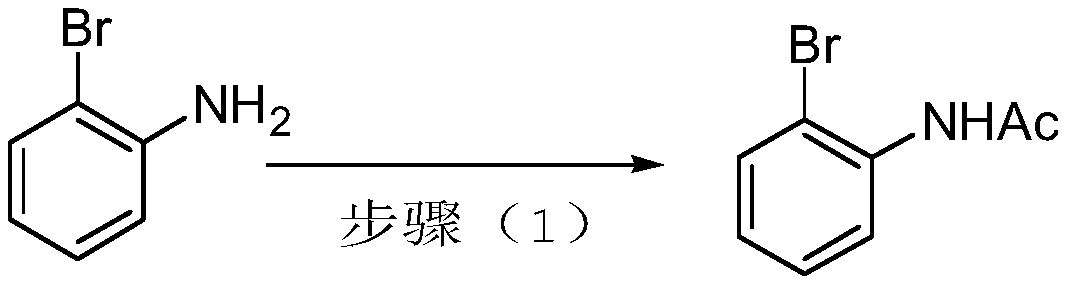
[0052] The preparation of embodiment 1 N-(2-bromophenyl) acetamide
[0053] Dichloromethane (260 mL) and 2-bromoaniline (86 g, 0.5 mol) were added to a 1 L reaction flask. Triethylamine (63.3 g, 0.63 mol) was added with stirring. Cool down to 0°C.
[0054] Acetyl chloride (42.7 mL, 0.6 mol) was added dropwise at 0°C. Keep the temperature at 0-10°C. 1h dropwise addition is complete. Rise to room temperature and stir for 4h.
[0055] Water (300 mL) and dichloromethane (200 mL) were added. Stir for 10min and separate the liquids. The aqueous phase was extracted once with dichloromethane (400 mL). Combine the organic phases. Wash with water (300mL), evaporate the organic phase to dryness under reduced pressure, add n-heptane (300mL) for recrystallization, and obtain solid N-(2-bromophenyl)acetamide (100.5g, yield 93.7%), mp=97 ~100°C, Rf=0.5 (PE:EA=2:1).
Embodiment 2
[0056] The preparation of embodiment 2 N-(2-bromophenyl) acetamide
[0057] Dichloromethane (260 mL) and 2-bromoaniline (86 g, 0.5 mol) were added to a 1 L reaction flask. Triethylamine (63.3 g, 0.63 mol) was added with stirring. Cool down to 0°C.
[0058] Acetyl chloride (42.7 mL, 0.6 mol) was added dropwise at 0°C. Keep the temperature at 0-10°C. 1h dropwise addition is complete. Rise to room temperature and stir for 4h.
[0059]Water (300 mL) and dichloromethane (200 mL) were added. Stir for 10min and separate the liquids. The aqueous phase was extracted once with dichloromethane (400 mL). Combine the organic phases. Wash with water (300mL), evaporate the organic phase to dryness under reduced pressure, add n-hexane (300mL) for recrystallization, filter, and blow dry to obtain solid N-(2-bromophenyl)acetamide (99.2g, yield 92.7% ), mp=97~100°C, Rf=0.5 (PE:EA=2:1).
Embodiment 3
[0060] The preparation of embodiment 3 N-(2-bromophenyl) acetamide
[0061] Dichloromethane (260 mL) and 2-bromoaniline (86 g, 0.5 mol) were added to a 1 L reaction flask. Triethylamine (63.3 g, 0.63 mol) was added with stirring. Cool down to 0°C.
[0062] Acetyl chloride (99.2 mL, 1.40 mol) was added dropwise at 0°C. Keep the temperature at 0-10°C. 1h dropwise addition is complete. Rise to room temperature and stir for 4h.
[0063] Water (300 mL) and dichloromethane (200 mL) were added. Stir for 10min and separate the liquids. The aqueous phase was extracted once with dichloromethane (400 mL). Combine the organic phases. Wash with water (300mL), evaporate the organic phase to dryness under reduced pressure, add cyclohexane (300mL) for recrystallization, and obtain solid N-(2-bromophenyl)acetamide (102.0g, yield 95.3%), mp=97 ~100°C, Rf=0.5 (PE:EA=2:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com