Green synthesis method of 3-phenanthridinyl propyl formate compound
A technology for phenanthridine propyl formate and green synthesis, which is applied in the field of synthesis of phenanthridine and its derivatives, can solve the problems of not being universally applicable, difficult to obtain reaction raw materials, harsh reaction conditions, etc., to avoid catalysts and additives The effect of easy use, easy product and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
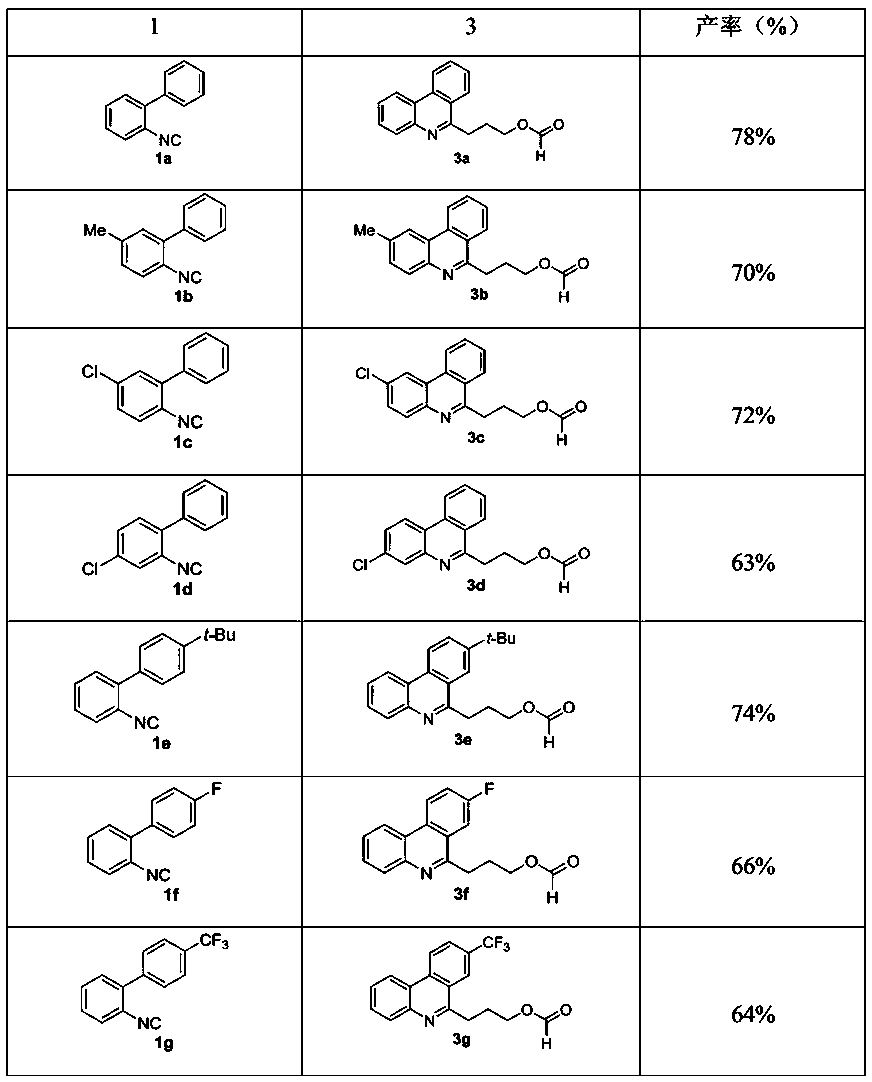
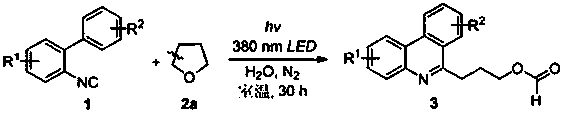
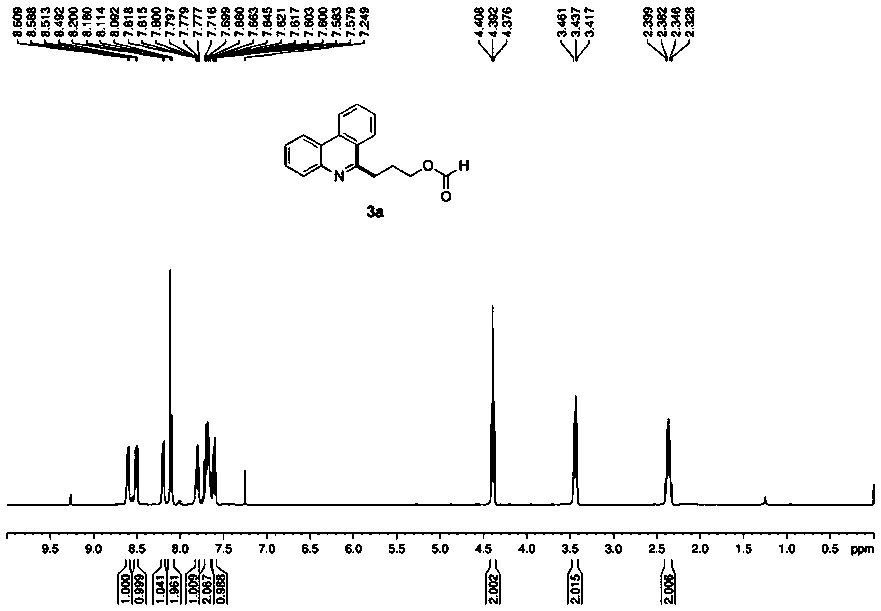
[0032] Such as figure 1 A green synthetic method of a shown 3-phenanthridine propyl carboxylate compound, the method may further comprise the steps:
[0033] (1) Accurately weigh 2-biphenylisonitrile (1a, 44.5 mg), and then add it into a reaction tube containing 2.0 mL of tetrahydrofuran and water (10:1);
[0034] (2) Place the reaction tube in step (1) on a 3W 380 nm LED lamp, and irradiate the reaction for 30 hours at room temperature. Under light irradiation, tetrahydrofuran selectively breaks the carbon-carbon bond;
[0035] (3) After the reaction, pour the reaction mixture into a separatory funnel filled with 10.0 mL of deionized water, extract and wash with ethyl acetate three times, 5.0 mL each time, and combine the organic phases;
[0036] (4) drying the organic phase obtained in step (3) with magnesium sulfate, suction filtration, removing magnesium sulfate, and concentrating the organic phase with a rotary evaporator to obtain a residue;
[0037] (5) The residue wa...
Embodiment 2
[0041] The difference from the preparation method in Example 1 is that the reaction raw material 1a is changed to 1b (2-isocyano-5-methyl-1,1'biphenyl, 48.3 mg), and other conditions are the same as in Example 1.
[0042] Column chromatography (silica gel, mobile phase: petroleum ether / ethyl acetate (9:1)) gave the target product 3b (48.9 mg, yield: 70%) as a colorless solid.
[0043] Such as Figure 4 , 5 As shown, compound 3b is characterized as follows: 1 H NMR (400 MHz, CDCl 3 ) δ: 8.58 (d, J =8.0 Hz, 1H), 8.28 (s, 1H), 8.17 (d, J = 8.0Hz, 1H), 8.11 (s, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.78 (td, J = 8.0, 1.2 Hz,1H), 7.64 (td, J = 8.0, 0.8 Hz, 1H),7.51 (dd, J = 8.4, 1.6 Hz, 1H), 4.38 (t, J = 6.4 Hz, 2H), 3.41 (t, J= 7.6Hz, 2H), 2.59 (s, 3H), 2.38-2.31 (m, 2H); 13 C NMR (100 MHz, CDCl 3 ) δ: 161.1, 159.1, 141.9, 136.2, 132.6, 130.2, 130.1, 129.3, 127.1, 125.6, 125.1, 123.4, 122.4, 121.5, 63.8, 31.7, 27.3, 21.8. HRMS (ESI) ([M+H] + ) calculated for C 18 H17NO 2 ...
Embodiment 3
[0045] The difference from the preparation method in Example 1 is that the reaction raw material 1a is changed to 1c (2-isocyano-5-chloro-1,1’biphenyl, 53.3 mg), and other conditions are the same as in Example 1.
[0046] Column chromatography (silica gel, mobile phase: petroleum ether / ethyl acetate (9:1)) gave the target product 3c (53.8 mg, yield: 72%) as a colorless solid.
[0047] Such as Figure 6 , 7 As shown, compound 3c is characterized as follows: 1 H NMR (400 MHz, CDCl 3 ) δ: 8.44 (d, J =8.0 Hz, 1H), 8.38 (s, 1H), 8.16 (d, J = 8.0 Hz, 1H), 8.12 (s, 1H), 7.97 (d, J = 8.4 Hz, 1H), 7.79 (t, J = 7.2 Hz, 1H), 7.68 (t, J = 7.6 Hz, 1H), 7.60 (d, J = 8.8 Hz, 1H), 4.39 (t, J = 6.0 Hz, 2H), 3.39 (t, J = 7.6 Hz, 2H), 2.38-2.31(m, 2H); 13 C NMR (100 MHz, CDCl 3 ( + ) calculated for C 17 H15ClNO 2 : 300.0786, found: 300.0788.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com