A fusion expression method of aep cyclase in Escherichia coli, a method for identifying the cyclization ability of aep cyclase and its application
A technology of fusion expression and Escherichia coli, applied in the field of genetic engineering, can solve the problem that the expression level cannot meet industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
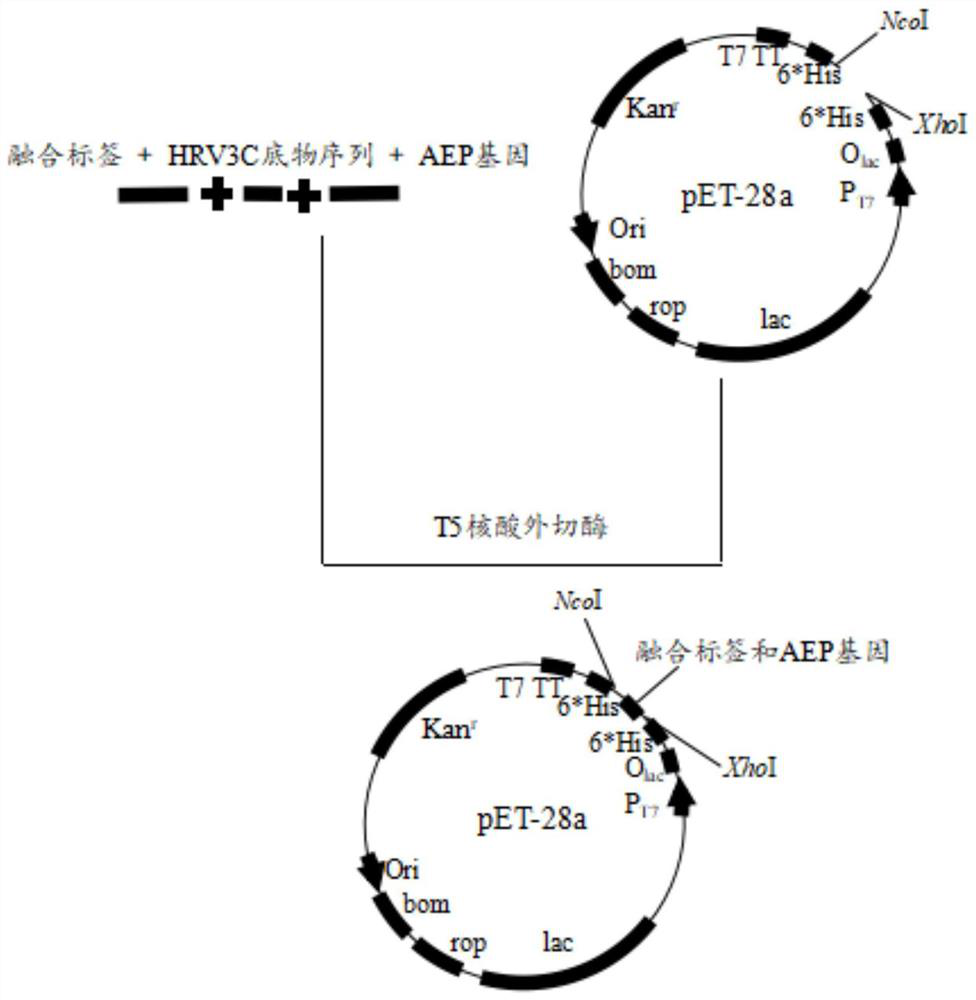
[0033] Embodiment 1 Fusion expression of AEP cyclase in Escherichia coli
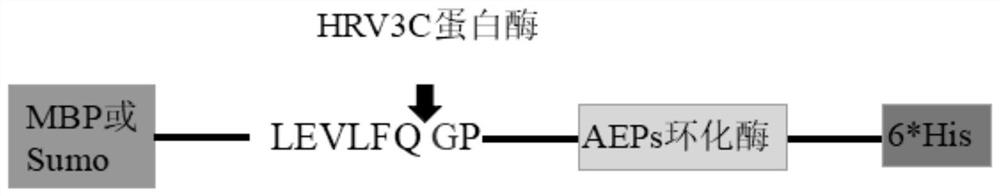
[0034] In this example, five fusion tags of MBP, NusA, SUMO, GFP, and Ubiquitin were used to fuse and express AEP, and vectors containing corresponding fusion tags were constructed, which were pET28a-GFP-AEP-6*His, pET28a-MBP-AEP- 6*His, pET28a-SUMO-AEP-6*His, pET28a-NusA-AEP-6*His, and pET28a-Ubiquitin-AEP-6*His. The specific experimental plan is as follows:
[0035] S1: Link the AEP sequence containing the protease polypeptide substrate recognition sequence with the fusion tag to construct a recombinant DNA sequence.
[0036] Use the AEP fragment synthesized by the gene as a template for PCR amplification. The sequence of the AEP fragment is shown in SEQ ID NO.16. The total PCR system is 100 μL, including 10 μL of 10×pfu Buffer, 4 μL of dNTPs (10 mmol / L), forward primer and reverse 4 μL of each primer, plasmid as template: 20-30ng, finally add 4 μL of pfu DNA polymerase, and finally add water to mak...
Embodiment 2
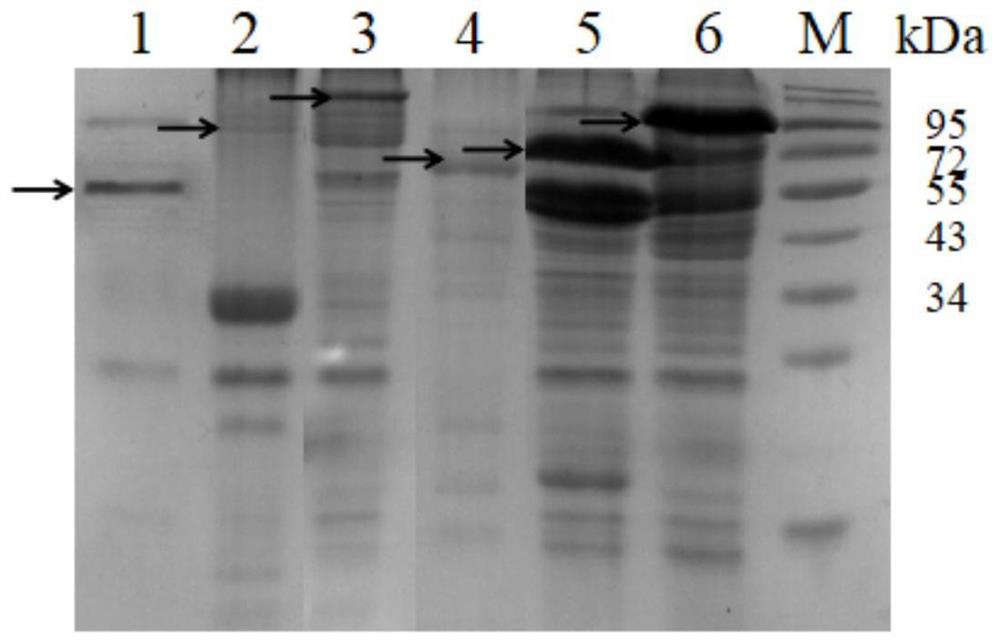
[0078] Example 2 Identification of cyclization ability of AEP cyclase
[0079] Step 1: Obtain the cyclization substrate of AEP cyclase
[0080] Since the SUMO protein is 11.0kDa in size, it can be well observed in SDS-PAGE and has the possibility of cyclization, so it is used as the cyclization substrate of AEP cyclase. Add GGGGSGGGGS to the N-terminal of the SUMO protease substrate, and add a longer Linker1 (GSGS), TEV protease substrate sequence (ENLYFQ / S), and a longer Linker2 (DVGGGGSEGGGSGGPGSGGEGSAGGGSAGGGS) to the C-terminal of the SUMO protease substrate in sequence and 6*His(HHHHHH), and finally add the recognition sequence of AEP cyclase at both ends to form a tandem substrate (GLP-SUMO protease substrate-Linker1-TEV protease substrate-Linker2-STRN / GLP-6*His ), the recognition sequence of AEP cyclase is shown in SEQ ID NO:13. Tandem substrates were constructed by overlap extension PCR, and the primer sequences were as follows:
[0081] SUMO-TEV-F: aactttaagaaggaga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com