Intraocular drug delivery device and associated methods
An eye and copolymer technology, applied in the field of ophthalmology, can solve problems such as poor drug permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Standard clear phacoemulsification and intraocular lens (Acrysof SA60AT; Alcon) implantation were performed on 35 rabbits. At each surgery, an intraocular device containing the active agent was inserted into the lens capsule of each rabbit. Rabbits were divided into 4 groups according to the active agent in the intraocular device. The device is loaded with 5-15 mg of Avastin, Timolol, Brimonidine or Latanoprost. Each group was evaluated to determine intraocular device and lens stability, capsular fibrosis, and healing of the cataract wound and anterior segment. A subset of eyes were assessed weekly for inflammation for 4 weeks and harvested at 1 month for histopathological assessment of capsule and CDR integrity.
Embodiment 2
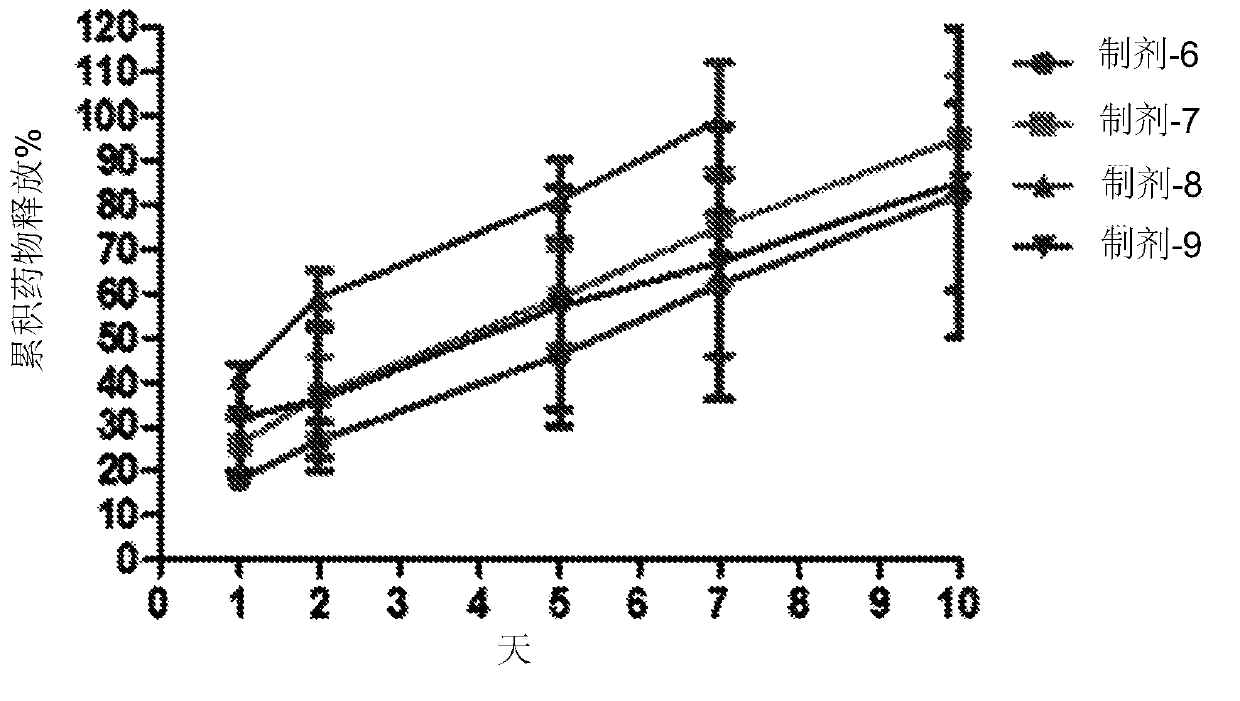
[0075] The procedure and setup described in Example 1 was repeated, except that aqueous humor and vitreous aspiration were performed biweekly, and drug concentrations were determined by HPLC and / or ELISA. In each drug group, half of the eyes were harvested at one month and the other half at two months. This was done as follows: Immediately after the rabbit was sacrificed and the eye was enucleated, the eye was frozen in liquid nitrogen to prevent perturbation and redistribution of the drug in the ocular tissue. Eyes were then sectioned in triplicate (aqueous humor, vitreous, and retina / choroid layers) to assess anatomic toxicity and tissue drug concentrations. Retrieve the intraocular device and assess the amount of remaining drug. Compare the distribution curve of intraocular device with 2.5mg / 0.1cc conventional intravitreal injections in order to directly compare different delivery methods.
[0076] At 2 and 4 months, rabbit eyes from the remaining subgroups were enuclea...
Embodiment 3
[0078] Following lens extraction (phacoemulsification), three intraocular devices were implanted in the eyes of New Zealand White rabbits under general anesthesia. Two of the devices were loaded with Avastin and one was loaded with the contrast agent Galbumin as a control. Proper intraocular device placement was verified by MRI and clinical examination.
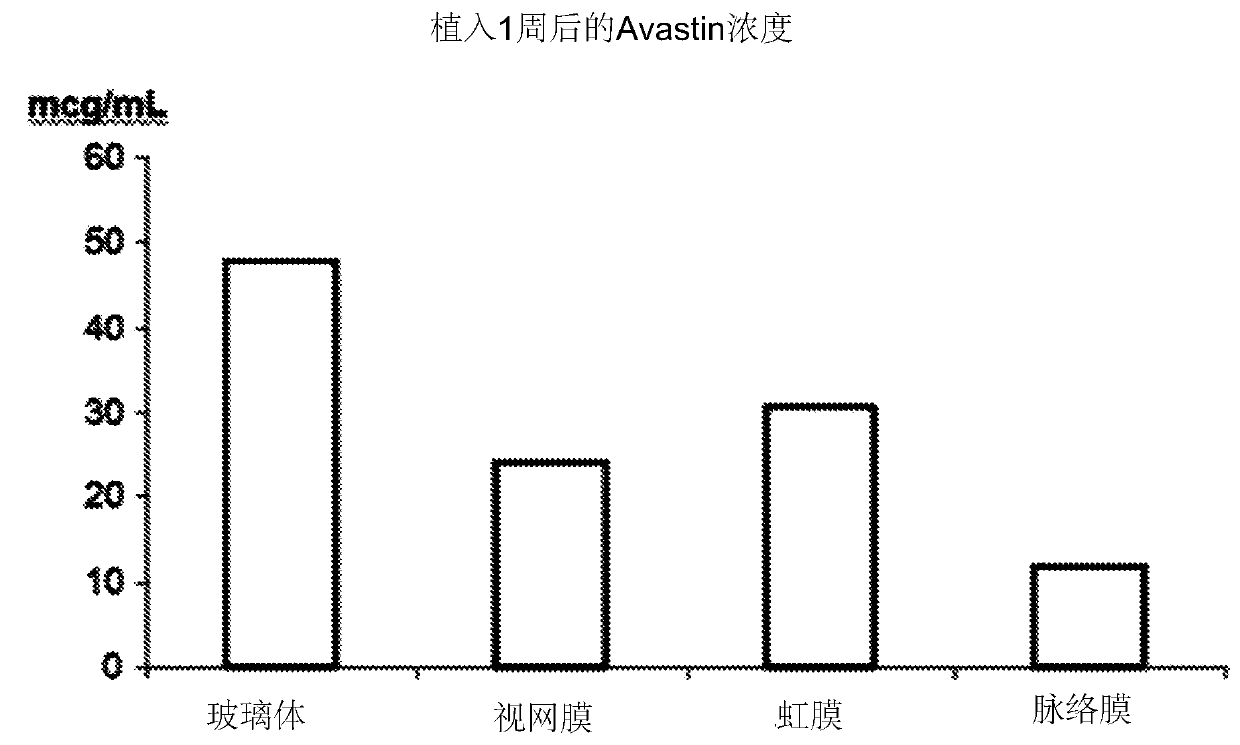
[0079] Rabbits were sacrificed 1 week after implantation and eyes were removed and assayed. Avastin concentrations of 24-48mcg / mL were detected by ELISA in the retina and vitreous, but not in control rabbit eyes. Figure 5 The amount of Avastin measured for each eye area at 1 week post-implantation is shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com